Preventive controls are part of a robust food safety management program that allows for a properly trained team to protect the food supply and the consumer. Some of the tools may include process preventive controls, allergen preventive controls, sanitation preventive controls, and supply chain preventive controls. The concepts of preventive controls can be used in almost any food manufacturing, packaging, storage, or distribution system.
Products that fall under preventive controls are:
- Ready-to-eat meals,
- Peanut butter,
- Power bars,
- Produce,
- Most regulated FDA food products.
- (Please get in touch with us for the complete list)
If a product is produced and distributed only in a single State, the producer can choose between a HACCP plan and a PC Plan, but since the product is not crossing State lines, it is regulated by the State government. Certain products have regulations, just to name a few: seafood, juice, USDA-regulated products, and low-acid canned foods.
Why set up Preventive Controls?
The beginning of the Preventive Controls Manual states:
“The Current Good Manufacturing Practice, Hazard Analysis, and Risk-based Preventive Controls for Human Food regulation (hereafter referred to as the Preventive Controls for Human Food regulation) was published on September 17, 2015 and is intended to ensure safe manufacturing/processing, packing and holding of food products for human consumption in the United States. The regulation requires that certain activities must be completed by a “preventive controls qualified individual” who has “successfully completed training in the development and application of risk‐based preventive controls at least equivalent to that received under a standardized curriculum recognized as adequate by FDA or be otherwise qualified through job experience to develop and apply a food safety system.”
The ultimate goal is to have a set of repeatable guidelines that keep our food supply safe. There are exemptions to the rule, but the overall methodology is to have a checklist to make sure we are looking at every potential hazard that is reasonably likely to occur.
So you will see similar terms as we are building preventive controls:
- Hazard
- Controls
- Monitoring
- Training
Every food safety plan starts with a foundation of Good Manufacturing Practices (GMPs), Standard Sanitation Operating Procedures (SSOPs), management commitment, education, and best practices. We can also include good agricultural and distribution practices and other programs that help keep the environment clean for food manufacturing, processing, and logistics.
Preventive Controls provide a system intended to be more flexible while, as always, ensuring product safety.
Who Is the PCQI and Why Are They Critical to Preventive Controls?
A Preventive Controls Qualified Individual (PCQI) is responsible for developing, implementing, and overseeing a facility’s food safety plan under the Preventive Controls for Human Food rule. This individual plays a central role in ensuring that hazards are correctly identified, preventive controls are effectively applied, and the food safety system remains compliant and functional.
To qualify as a PCQI, a person must either complete standardized training recognized by the FDA, such as the curriculum developed by the Food Safety Preventive Controls Alliance (FSPCA), or gain equivalent experience through their role in food manufacturing. Regardless of the pathway, the PCQI must demonstrate a deep understanding of hazard analysis, risk-based preventive controls, monitoring procedures, verification activities, and corrective actions.
The PCQI’s responsibilities extend beyond initial plan development. They are also responsible for validating that preventive controls are adequate, reviewing records, verifying implementation, and adjusting the plan in response to process changes or audit findings.
In short, the PCQI ensures that the facility’s food safety system is compliant on paper and functioning in real-time to protect public health.
Start Your Preventive Controls Plan Today
Don’t wait for a compliance issue to arise. Use FoodReady’s templates and expert guidance to build your food safety plan now.
Understanding Each Type of Preventive Control
Preventive controls are designed to reduce the risk of foodborne illness by targeting the specific points in a process where hazards are most likely to occur. The Food Safety Modernization Act outlines four primary types of preventive controls that can be implemented depending on the nature of the product, facility, and identified hazards.
These include process, allergen, sanitation, and supply chain controls.
1. Process Preventive Controls
These controls are applied during manufacturing and are intended to manage hazards during specific operations. This might include time and temperature parameters for cooking, pH levels in acidified foods, or filtration to remove physical contaminants.
For example, a soup manufacturer may implement cooking temperature controls to eliminate Salmonella in poultry-based products.
2. Allergen Preventive Controls
Allergen controls help prevent cross-contact between allergenic and non-allergenic products. This includes dedicated lines or equipment, validated cleaning procedures between product changeovers, and accurate ingredient labeling. A facility producing peanut-containing and peanut-free snack bars requires strict allergen controls to protect consumers with allergies.
3. Sanitation Preventive Controls
These controls ensure food-contact surfaces and the production environment are kept clean to reduce microbiological hazards. Examples include regular cleaning and sanitizing of equipment, monitoring sanitizer concentrations, and verifying effectiveness through environmental testing.
For instance, a facility producing ready-to-eat salads may implement sanitation procedures to control Listeria monocytogenes.
4. Supply Chain Preventive Controls
When a facility relies on ingredients or materials from suppliers that carry known risks, supply chain controls are used to ensure safety. This may involve requiring certificates of analysis (COAs), conducting supplier audits, or verifying that incoming goods meet food safety standards.
A company sourcing raw spices, often prone to microbial contamination, might implement a supply chain verification program to ensure pathogen control at the source.
Each of these controls plays a critical role in developing a facility-specific food safety plan that is proactive, science-based, and aligned with regulatory expectations.
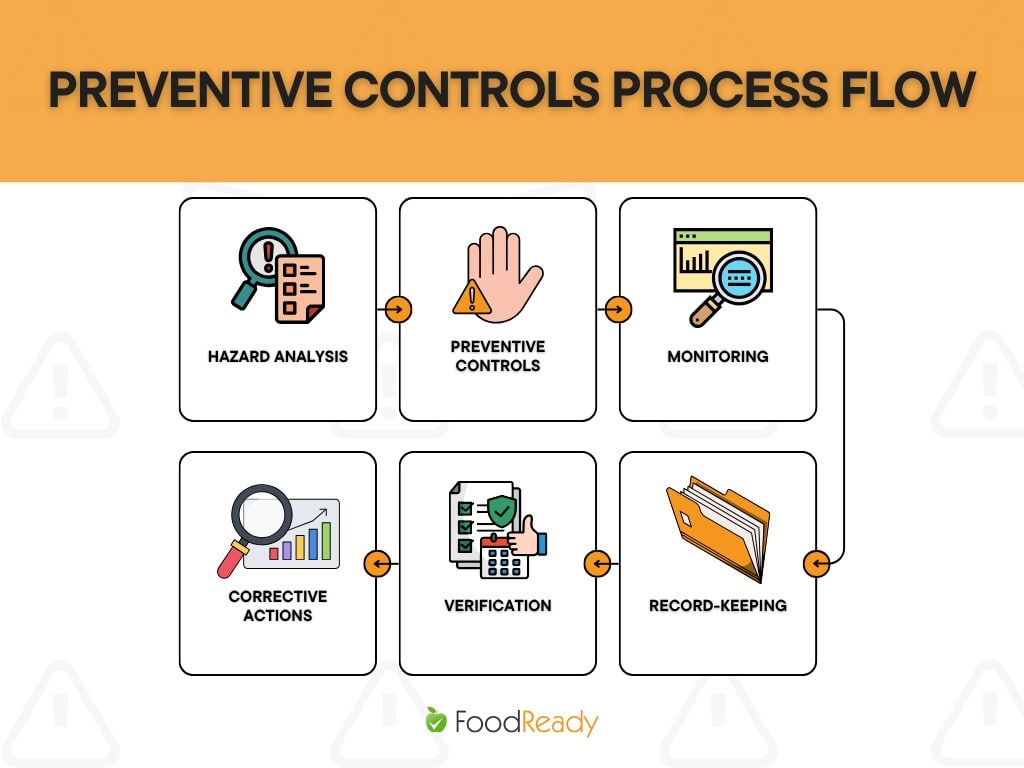
Preventive Controls Process
Developing a preventive controls-based food safety plan involves more than identifying general risks; it requires a structured, science-driven process tailored to each facility’s specific hazards, operations, and products.
The goal is to prevent foodborne illness by establishing proactive, verifiable systems.
Here’s how the process unfolds:
1. Conduct a Hazard Analysis
The first step is to evaluate your ingredients, processes, and facility environment to identify known or reasonably foreseeable biological, chemical, and physical hazards.
Only significant hazards likely to cause illness or injury if not controlled require preventive controls.
2. Establish Preventive Controls
Once hazards are identified, preventive controls are implemented to eliminate or reduce those hazards to acceptable levels. Depending on where the risk originates, these may include process controls (e.g., time/temperature), allergen controls, sanitation controls, or supply chain controls.
3. Define Parameters and Monitoring Procedures
Each preventive control must include defined limits or conditions (e.g., minimum cook temperature, acceptable pH level). Monitoring activities are then implemented to ensure these conditions are consistently met during production.
4. Plan Corrective Actions
Corrective actions must be taken promptly if a deviation occurs, such as a failure to meet a critical temperature or the discovery of an allergen cross-contact. These actions should be predefined, including steps to identify and correct the issue, evaluate the affected product, and prevent recurrence.
5. Verify Effectiveness
Verification ensures the food safety system is functioning as intended. This includes reviewing monitoring records, conducting internal audits, performing environmental testing, and validating that controls can control the identified hazards.
6. Maintain Records
All preventive control activities must be documented. Records prove that the system works, supports regulatory compliance, and helps management, auditors, and inspectors evaluate overall food safety performance.
By following these six steps, facilities can build a preventive controls program that is systematic, flexible, and compliant with FSMA expectations, ensuring food is produced, packed, and held in a way that protects public health.
Preventive Controls vs. HACCP: What’s the Difference?
While the Preventive Controls (PC) rule builds on the foundation of the traditional HACCP (Hazard Analysis and Critical Control Points) system, the two frameworks differ in scope, regulatory structure, and application. Both aim to prevent food safety hazards, but PC provides a broader and more flexible approach aligned with the requirements of the Food Safety Modernization Act (FSMA).
Below is a comparison of key elements to help distinguish the two systems:
| Feature | HACCP | Preventive Controls (PC Rule) |
|---|---|---|
| Regulatory Basis | Codex Alimentarius / USDA / FDA (for select industries) | FSMA – FDA regulation for most human food facilities |
| Applies To | Juice, seafood, meat, poultry, and low-acid canned foods | Most FDA-regulated food products (except those with specific rules) |
| Required by | USDA and FDA (product-specific) | FDA (under FSMA) |
| Control Types | Focus on Critical Control Points (CCPs) | Includes Process, Allergen, Sanitation, and Supply Chain controls |
| Flexibility | Structured, process-focused | More flexible; includes management-based and facility-specific controls |
| Plan Developer Requirements | Must be trained in HACCP principles | Must be a Preventive Controls Qualified Individual (PCQI) |
| Corrective Action & Verification | Required | Required, plus additional documentation and reanalysis triggers |
| Record-Keeping | Essential for CCPs and monitoring | Comprehensive recordkeeping, including non-CCP activities |
| Environmental Monitoring | Not always required | Required for RTE products exposed to the environment |
In practice, HACCP and Preventive Controls share the same core objective: proactively identifying and managing food safety hazards.
However, the Preventive Controls rule expands beyond critical control points, incorporating a more holistic view of the facility, supply chain, and preventive programs needed to ensure product safety.
Wrapping-up
Preventive controls are vital for ensuring food safety, covering areas like process, allergen, sanitation, and supply chain management. These controls, mandated by the Food Safety Modernization Act, require a systematic, science-based approach to prevent foodborne illnesses. They apply to a variety of products, including ready-to-eat meals, peanut butter, and produce.
Implementing preventive controls involves conducting hazard analyses, establishing measures, and monitoring compliance. This approach ensures products are produced safely and helps maintain a clean environment for food processing.
For effective implementation, good manufacturing practices (GMPs) and standard sanitation procedures (SSOPs) are essential. Facilities can benefit from FoodReady Plan Templates and expert support to develop robust food safety plans.
Get started with your food safety documentation today. Contact FoodReady for templates and expert assistance!
FAQs
Not all facilities are required to implement preventive controls. Tiny businesses may qualify for modified requirements, and certain products, like juice, seafood, meat, poultry, and low-acid canned foods, are regulated under separate HACCP-based rules. However, most FDA-regulated food facilities must comply with the Preventive Controls for Human Food rule.
A hazard requiring a preventive control is “reasonably likely to cause illness or injury in the absence of control.” Hazards not meeting this threshold may still require good manufacturing practices (GMPs), but do not trigger the need for a full preventive control measure.
Yes, as long as the individual meets the training or experience requirements and has sufficient knowledge of each facility’s operations to develop and oversee the food safety plans effectively. However, the PCQI must be actively involved in implementing and verifying the plan.
Templates for hazard analysis, monitoring logs, corrective action records, and validation checklists can streamline compliance. FoodReady offers pre-built plan templates and expert support tailored to FDA expectations, making implementation easier for facilities of all sizes.






