FDA Consultant
Our FDA Consultants Help You Develop & Implement FDA-Compliant Food Safety Programs & Prepare for a Successful FDA Audit.
- Expert guidance on FDA Compliance & FSMA Compliance, including FDA 483 notices and warning letters and recall management.
- Writing of FDA-compliant Food Safety Plans (Preventive Control), HACCP, SOPs, Environmental Monitoring Programs, & Sanitation Procedures.
- Full-service support for developing and implementing FDA-compliant Food Safety Programs, including training on FDA Requirements.
- End-to-end FDA Documentation Management and inspection readiness, with fast-track to FDA Approvals and adherence to Global Regulatory Standards.
- Customized solutions for FDA Inspections, Compliance Audits, and Risk Mitigation.

Leading Companies Trust FoodReady






Meet the Lead of Our Expert FDA Consultant Team
Our Chief Food Safety Officer, Dave Seddon, is a longtime food safety auditor who has audited over 500 manufacturers for SQF certification, as well as GMP certification. Dave was named a finalist for the SQF auditor of the year by the SQF Institute.

Engage Our FDA Consultants to Guarantee FDA Compliance.
We will deliver the best job with the best team
Faster FDA Approval:
Our FDA consultant will streamline your processes with expert guidance to reduce errors and speed up FDA Compliance.
Tailored Compliance Plan:
Our FDA consultant will provide customized roadmaps based on your specific product and regulatory needs.
Error & Delay Prevention:
Our FDA Consultant will help you avoid costly mistakes through accurate documentation and regulatory insight.
Inspection Readiness:
Our FDA consultant will prepare your facilities and staff to pass FDA inspections smoothly.
Market Access & Growth:
Our FDA compliance consultant will enable legal U.S. market entry and build credibility for your business expansion.

Hear It From Real People
Grow Your Food Business With Our
FDA Consulting Services
We’ll schedule and customize your program, review documentation, and develop FDA compliance plans and checklists. We can also perform a gap assessment to identify areas for improvement and create necessary documentation for a successful audit.
Trust us to make your audit process a breeze with our FDA consulting services. Do you need an FDA compliance plan as part of a food safety Compliance? Our FDA compliance consultants will work with your business until you achieve your FDA Compliance.
Get Your FDA Approval Plan Up and Running.
Our team of FDA 483-certified and experienced food safety professionals excels at quickly and efficiently establishing and implementing your FDA compliance plan to meet FDA standards!
Save time and skip the hassle of doing it yourself. Trust our experts to customize a food safety solution tailored to meet your unique needs and FDA compliance requirements by achieving:
- Improved food safety and product quality
- Compliance with FDA regulations and standards
- Reduced risk of FDA non-compliance, FDA 483, fines, and penalties
- Improved FDA risk management and hazard control
- Customized food safety program designed to meet your specific needs and goals

We Assist You With Implementing Your FDA 483 Program
We’ll team up with you to actualize your FDA 483 approval program, ensuring you ace your next FDA compliance inspection.
But wait, there’s more! Not only do we offer top-notch FDA regulatory compliance consulting to support you, but we’ve also got a user-friendly mobile app that makes managing all your HACCP Plans and FDA food safety records effortless. Stay on top of everything from checklists to traceability and environmental monitoring — all at your fingertips!

Why Use FoodReady AI?
With searchable archives of temperature logs, cleaning records, and SOPs.
With automated compliance reminders and deviation tracking.
With signatures, access control, and versioning fully aligned with FDA standards.
How Can Our AI Food Safety Management System Help You?
Our AI-Powered Food Safety Management System is more than just a compliance tool – it’s an intelligent system designed to streamline and strengthen your food safety and operational processes. The AI gives you great suggestions, and it’s all built-in with food safety smart tips from experts tailored for different kinds of food industries.
Rapid AI HACCP Plan Creation
FoodReady’s AI HACCP Builder offers pre-built templates, cutting HACCP plan development from hours to minutes.
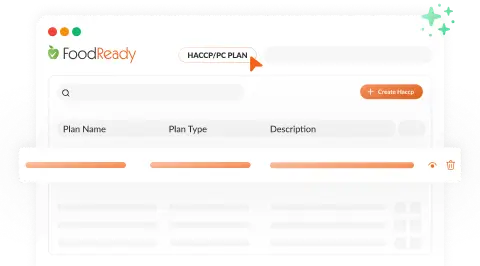
AI-Generated SOPs & Checklists
FoodReady’s AI Assistant creates and automates SOPs and checklists, detecting missed tasks and ensuring protocol compliance
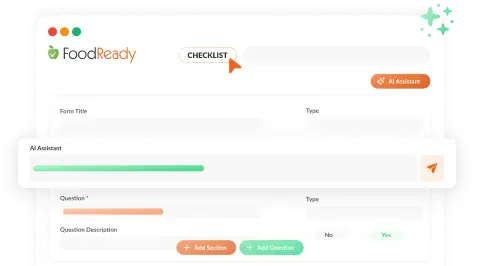
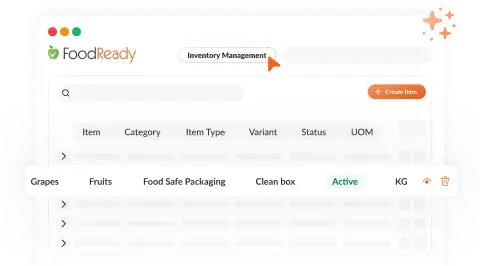
Unified Batch & Inventory Management
Our AI Assistant offers real-time tracking of production, inventory, and costs, enhancing efficiency and reducing compliance problems
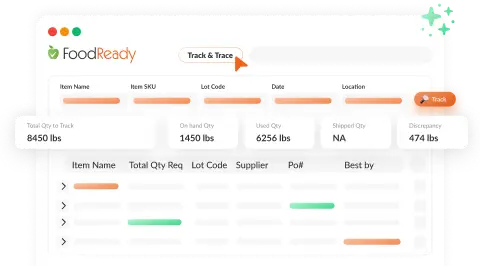
Complete Traceability & Recall Readiness
FoodReady’s AI provides full traceability by linking ingredients to final products, facilitating rapid recalls and efficient audits
Automated Supplier Compliance
Reduce manual effort and increase transparency with AI-powered automatic management and updates of supplier documents
Live Monitoring & Instant Alerts
AI-powered dashboards offer real-time KPI monitoring and safety incident alerts for timely intervention.
Our Process For Helping You Obtain FDA Approval



Gap Assessment
This step is where we request and review the client’s existing documentation and facility and identify where the gaps exist and how to close the gaps to pass a successful FDA inspection.
Food Safety Document Development
Environmental Monitoring Program
This step is where we develop your environmental monitoring program, including Listeria detection. FoodReady also has an easy-to-use mobile app that makes it easier to manage your environmental monitoring program.
We Can Help You Develop Your Food Safety Documentation
- Eliminate Paperwork.
- Increase Efficiency.
- Reduce Potential Hazards.
- Minimize Operational Risks.
- Train New Staff Faster.

Frequently Asked Questions
An FDA Consultant advises food manufacturers, processors, and distributors on complying with U.S. Food and Drug Administration (FDA) regulations. This includes assistance with labeling, facility registration, FSMA compliance, food recalls, and import/export documentation. Their expertise helps ensure FDA approval or avoid penalties during inspections.
An FDA 483 notice is issued after an inspection when the FDA identifies conditions or practices that may violate regulatory requirements. An experienced consultant can help analyze the notice, develop corrective action plans, and implement changes to ensure compliance.
While FSMA (Food Safety Modernization Act) requirements are a subset of FDA regulations focused on preventive controls, FDA requirements encompass broader guidelines, including labeling, sanitation, and traceability, applicable to all food manufacturers.
FDA approval is essential for industries involved in food manufacturing, processing, packaging, and distribution, including specialized sectors like dietary supplements, pet foods, and beverages.
Yes, FDA consultants can align your programs with international standards such as ISO 22000 or Codex Alimentarius, ensuring compliance for businesses operating across borders.
Common issues include inadequate sanitation practices, incomplete or missing documentation, insufficient traceability programs, and failure to comply with labeling or allergen controls. A consultant can help address these areas proactively.

Speak with our
Expert Consultants
Get a Free Consultation With Our Food Safety
and Quality Experts Today!
Get in Touch with Us
We are always here to help

