When it comes to food safety, you’ve probably heard of ‘cGMP’ – it’s everywhere in the industry.
But what exactly is it, and why does it matter so much?
This guide is for all producers and quality pros in the food industry to learn about current Good Manufacturing Practices (cGMP).
Initially, we’ll guide you through establishing and upholding cGMP in your organization to ensure product excellence. Then, we’ll demonstrate how these measures contribute to building trust among the public.
Come along as we explore one of the critical elements of food production in the 21st century.
What is cGMP?
Current Good Manufacturing Practices (cGMP) are FDA-enforced regulations ensuring the quality, safety, and efficacy of food products, pharmaceuticals, and medical devices. cGMP minimizes risks of contamination, adulteration, and mislabeling by maintaining hygienic facilities, controlled environments, trained personnel, and strict documentation.
Unlike GMP, cGMP requires ongoing updates to reflect modern technologies and evolving industry standards, ensuring continuous improvement and compliance in manufacturing processes.
Why is cGMP Important for Food Production?
The value of cGMP lies in consumer protection. It’s a quality assurance framework that, when adhered to, helps prevent unsanitary conditions in food manufacturing, ensures proper employee training, and covers everything from raw materials to the final product. cGMP is the backbone of a transparent and sustainable food production system, contributing to public safety and trust.
It doesn’t only stop at safety, though. cGMP also significantly affects a business’s reputation and bottom line.
Adhering to cGMP boosts consumer confidence, reduces recalls and liability issues, enhances brand value, facilitates international trade, improves efficiency and customer satisfaction, and ensures regulatory compliance.

As we can see, cGMP isn’t just a legal or regulatory requirement; it’s a standard practice that sets a precedent for the industry and defines the seriousness with which a company addresses public health.
Difference Between GMP and cGMP
You might have heard the term ‘GMP’ interchangeably with ‘cGMP.’
While they share a fundamental purpose of maintaining quality, they are distinct in their focus.
Good Manufacturing Practices (GMP) are a set of regulations that are more generic and apply to all food products and food processing steps. On the other hand, cGMP is specific to food products but is also linked to sectors like pharmaceuticals, dietary supplements, and cosmetics.
The ‘c’ in cGMP stands for ‘current,’ indicating that the most recent technologies and systems are in place to guarantee that the end product is not just adequately safe for consumption, but is also effective and bears the necessary purity characteristics it is represented with.
Read our detailed comparison of cGMP vs. GMP to learn more about what sets cGMP apart from GMP.
Which Sectors and Products Are in the Scope of cGMP?
The scope of cGMP spans a diverse range of industries and sectors, reflecting its pivotal role in ensuring the production of safe, high-quality products.
cGMP’s influence extends to pharmaceuticals, dietary supplements, cosmetics, food manufacturing, and more.
This wide applicability underscores the universal need for stringent quality control and safety standards across all sectors impacting public health.
Industries and Sectors Covered
cGMP regulations are not limited to a single industry. They permeate several sectors that are integral to public wellness:
- Food Manufacturing: cGMP lays the foundation for sanitary and quality-controlled food production processes.
- Pharmaceuticals: Ensuring the safety, potency, and purity of medications.
- Dietary Supplements: Regulating the production and labeling of supplements to guarantee their quality.
- Cosmetics: Overseeing the manufacturing and labeling of cosmetic products to ensure they meet safety standards.
Types of Products Affected
cGMP standards impact a broad array of products within these sectors, from the food on our tables and the supplements in our cabinets to the medications we rely on and the cosmetics we use daily. This widespread influence is a testament to cGMP’s crucial role in product safety and consumer trust.
Application Across the Product Lifecycle
cGMP’s reach extends through the entire product lifecycle:
| Design and Development | Ensuring products are designed with safety and quality in mind from the outset. | |
| Procurement of Raw Materials | Regulating the quality and handling of raw materials to avoid contamination and ensure purity. | |
| Manufacturing Processes | Implementing strict procedures and controls at every production stage to maintain consistent quality. | |
| Packaging and Labeling | Ensuring products are accurately labeled and packaged to maintain their quality and safety. | |
| Storage and Distribution | Overseeing the conditions in which products are stored and distributed to prevent spoilage or damage. |
This comprehensive application emphasizes cGMP’s role in safeguarding the end product and ensuring ethical and safe practices throughout the production and distribution processes.
What are the Key Principles of cGMP
cGMP, or Current Good Manufacturing Practices, encompasses a broad range of principles to ensure products are consistently produced and controlled according to quality standards.
These principles cover everything from the design of facilities and equipment to how personnel are trained.
Here’s a closer look at the key principles of cGMP:
1. Quality Management Systems
At the heart of cGMP is the Quality Management System (QMS), designed to oversee and control all aspects of the production process. This system confirms that a structured process is in place for managing risk, improving product quality, and ensuring customer satisfaction. It encompasses the organization’s structure, procedures, processes, and resources needed to implement quality management.
2. Personnel Qualifications and Training
A critical principle of cGMP is ensuring that all personnel involved in the manufacturing process are qualified and adequately trained. This includes regular training programs to keep staff updated on the latest quality control procedures and standards, enhancing the workforce’s skills to prevent errors and maintain high-quality production.
3. Facility and Equipment Design
The design and maintenance of facilities and equipment play a crucial role in cGMP. Facilities must be designed to minimize the risk of contamination or errors, including proper airflow, temperature controls, and clean areas. Equipment must be maintained to a high standard, with regular checks to ensure it operates correctly and does not compromise product quality.
4. Control of Materials
cGMP requires strict control over all materials used in manufacturing, from raw materials to finished products. This includes verifying the quality of incoming materials, proper storage to prevent contamination, and tracing materials throughout the production process to ensure consistency and quality.
5. Production and Process Controls
Adequate production and process controls are essential to maintain the integrity of the manufacturing process under cGMP. This involves establishing clear, written procedures for each process step, monitoring critical parameters, and implementing corrective actions when necessary to ensure the product meets its specifications.
6. Quality Control and Laboratory Testing
Quality control and laboratory testing are pivotal to verifying that products meet the required safety, quality, and efficacy standards. This includes testing raw materials, intermediate products, and the final product, using validated methods to ensure the accuracy and reliability of results.
7. Packaging, Labeling, and Distribution
cGMP principles extend to how products are packaged, labeled, and distributed. This ensures that products are packaged to maintain quality, correctly labeled to provide accurate information, and distributed in conditions that do not compromise their integrity.
8. Documentation and Record Keeping
Finally, comprehensive documentation and meticulous record-keeping are fundamental to cGMP. This includes keeping detailed records of every aspect of the manufacturing process, from the receipt of materials to the final distribution of products. Proper documentation ensures transparency, traceability, and accountability, allowing for thorough audits and inspections.
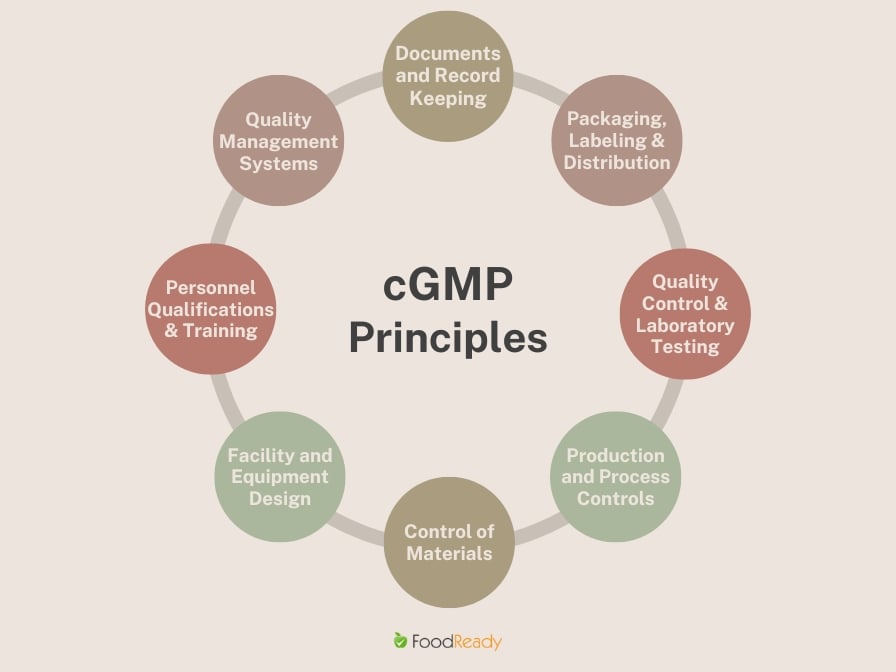
Together, these principles of cGMP form a framework that fosters high standards of quality, safety, and efficacy in the production of food, pharmaceuticals, dietary supplements, and cosmetics, protecting consumers and enhancing the credibility of products in the global market.
How Regulators Ensure Adherence to cGMP?
The Food and Drug Administration (FDA) and other international regulatory bodies carry out regular inspections and audits to ensure that manufacturers are compliant with cGMP. These inspections are comprehensive and focus on various aspects such as facility conditions, equipment maintenance, employee practices, and adherence to documented procedures.
The scope of the FDA’s inspections is extensive and involves the following steps:
- Pre-announcement to the facility to arrange for the inspection
- Entry review of facility records
- Inspection of the facility’s physical conditions
- Assessment of adherence to cGMP practices
- Review of the products’ manufacturing processes
Key FDA cGMP Regulations (21 CFR Title 21)
The FDA’s Current Good Manufacturing Practice (cGMP) regulations, found in Title 21 of the Code of Federal Regulations (CFR), are designed to ensure the safety, purity, and quality of various products by preventing them from being adulterated or misbranded as outlined in the Federal Food, Drug, and Cosmetic Act. These regulations apply to:
Pharmaceuticals and Drugs
21 CFR Part 210 – General cGMP requirements for manufacturing, processing, packing, and holding of drugs.
21 CFR Part 211 – cGMP standards for finished pharmaceuticals, including quality control, batch records, and labeling.
21 CFR Part 212 – cGMP for Positron Emission Tomography (PET) drugs, ensuring their safety and effectiveness.
21 CFR Part 314– Requirements for FDA drug approval (New Drug Applications – NDAs and Abbreviated New Drug Applications – ANDAs).
21 CFR Part 600 – Regulations for biological products, including vaccines, blood products, and biotechnology-based treatments.
Other FDA-Regulated Products
| Food Manufacturing | 21 CFR Part 117 focuses on preventive controls for human food. |
| Dietary Supplements | 21 CFR Part 111 ensures quality control in supplement manufacturing and labeling. |
| Medical Devices | 21 CFR Part 820 sets cGMP requirements for device design and production. |
Steps to Achieve cGMP Compliance in the Food Industry
Achieving and maintaining cGMP compliance is a challenging and ongoing process. However, businesses can adopt a structured approach to ensure that their operations adhere to these stringent requirements.
The steps to achieve compliance include:
- Conducting a comprehensive cGMP audit of your facility and processes
- Building a cGMP team to oversee the compliance process
- Develop and implement cGMP-compliant standard operating procedures (SOPs)
- Providing your employees with the necessary cGMP training
- Regularly reviewing and updating your cGMP documentation
- Establishing a continuous improvement process to enhance cGMP compliance
These measures empower businesses with the tools and knowledge to achieve cGMP compliance.

By doing so, they can ensure the production of safe and high-quality products that meet customer expectations and regulatory requirements.
Consequences of Non-Compliance with cGMP regulations
If an FDA inspection identifies cGMP violations, the agency may impose enforcement actions to ensure compliance and protect public health. These actions can include:
- FDA Form 483 – Official notice of non-compliance requiring corrective action.
- Warning Letters – Public documentation of serious cGMP violations.
- Product Recalls – Removal of non-compliant products from the market.
- Import Bans & Facility Shutdowns – Restricting operations of high-risk facilities.
In severe cases, fines, legal actions, or enforced product recalls may be imposed. To avoid regulatory sanctions, companies must establish a robust cGMP program, continuously monitor compliance, and implement proactive improvements in their quality management systems.
Importance of Documentation and Record-Keeping
Ensuring cGMP compliance entails meticulously documenting all activities and procedures throughout food production.
The records function as a detailed roadmap guiding a product from creation to consumption. They act as a tool for preserving the essence of each manufacturing event. In times of crisis, they serve as a beacon illuminating the path to product safety. During inspections, they provide a shield of compliance. These records drive progress by refining processes and unveiling a polished masterpiece.
The precision of record-keeping portrays a vivid image of a cGMP-compliant food manufacturing facility. Therefore, proper documentation and record-keeping are crucial for ensuring cGMP compliance and maintaining the highest quality standards in the food industry.
The ongoing efforts to achieve and maintain cGMP compliance benefit businesses and consumers, who can trust in the safety and efficacy of the products they consume.
Developing a Comprehensive Quality System Under cGMP
Implementing a comprehensive quality system is integral to cGMP compliance. This system should incorporate quality assurance (QA) and quality control (QC) and cover all aspects of the food production process – from raw material sourcing to final product release.
A robust quality system includes the following components:
- Quality policy and objectives
- Standard operating procedures (SOPs)
- Quality risk management
- Change control
- Deviation and CAPA systems
- Product complaints and recalls
By integrating these components into your quality system, you can ensure that all activities in your facility are aligned with cGMP requirements.
What is the Role of Quality Assurance (QA) and Quality Control (QC) in cGMP Compliance?
QA and QC play distinct but complementary roles in ensuring cGMP compliance. Quality Assurance is responsible for establishing and maintaining the quality of product development processes. This includes defining specifications, developing and implementing quality processes, approving and monitoring process validation activities, and conducting audits.
Quality Control, on the other hand, focuses on testing and ensuring the quality of raw materials, in-process samples, and finished products. QC activities involve inspection, testing, and sampling, and it is crucial for identifying deviations and ensuring that only high-quality products are released to the market.
QA and QC are critical for maintaining consistency, reliability, and compliance in food manufacturing operations.
Challenges and Solutions for cGMP Compliance in Food Manufacturing
cGMP compliance in food manufacturing presents several challenges, including managing complex supply chains, changing regulatory requirements, and adapting to new technologies and processes.
To overcome these challenges, companies can adopt risk-based approaches to quality management. For example, integrating advanced cGMP/GMP software for enhanced process monitoring and control and nurturing a culture of continuous improvement alongside empowering their employees.
Furthermore, engaging in industry-wide collaborations and sharing information has proven pivotal. By tackling these challenges directly, firms can ensure their cGMP compliance strategies are practical and current, safeguarding their commitment to quality and safety.
What are Some Best Practices for cGMP Training Programs?
An effective training program is vital for establishing and maintaining cGMP compliance. Training should be ongoing, with regular updates and refreshers to ensure all employees know the latest standards and requirements.
Best practices for cGMP training programs include:
- Tailoring training to the specific needs of different roles and responsibilities
- Using a variety of training methods, such as classroom instruction, e-learning, and practical demonstrations
- Providing opportunities for feedback and questions from trainees
- Assessing the effectiveness of training through testing and performance evaluation
When businesses invest in a thorough training program, they equip their employees with the knowledge and skills needed to meet and maintain cGMP compliance.
Common Challenges in Maintaining cGMP Compliance
Even after achieving cGMP compliance, businesses face ongoing challenges in maintaining these high standards. Some common challenges include:
- Employee turnover and the loss of institutional knowledge
- Ensuring the consistent application of cGMP requirements across all facility operations
- Meeting the increased scrutiny of regulatory expectations
- Keeping up with advancements and updates in cGMP guidelines
- Poor record-keeping and missing batch records hinder traceability
- Insufficient training leads to non-compliance with hygiene and safety protocols
- Ineffective cleaning practices increase contamination risks
- Using unverified raw materials jeopardizes product integrity
- Temperature deviations and cross-contamination threaten food safety
To address these challenges, businesses must remain vigilant, proactive, and committed to continuous improvement.
Maintaining cGMP compliance presents a continuous challenge for businesses in the food industry, even after initial certification is achieved.
Factors such as high employee turnover, which leads to a loss of critical knowledge, and the need for uniform application of cGMP standards across operations, complicate this task. Businesses must also stay agile, continuously updating their operations to meet the shifting demands of regulatory expectations and current cGMP guidelines.
To illustrate, consider the case of a prominent dairy producer faced with a significant cGMP compliance challenge. The company encountered issues related to inconsistent quality control measures across its facilities, which resulted in a costly recall of contaminated products.
This incident underscored the importance of stringent process monitoring and the application of cGMP standards. The company also consulted with an experienced cGMP consultant.
In response, the dairy producer implemented a comprehensive training program to reduce employee knowledge gaps. At the same time, it introduced advanced cGMP/GMP software to enhance process control and documentation.
This strategic approach helped the company regain compliance and reinforced the necessity of continuous vigilance and adaptation to meet cGMP standards.
What Challenges Do Companies Face with cGMP Compliance Across International Borders?
When comparing cGMP (Current Good Manufacturing Practices) standards worldwide, it becomes evident that while the core principles are universally recognized, specific requirements may vary significantly from one region to another.
The United States, the European Union, and Japan, among others, have established their cGMP guidelines tailored to ensure food products’ safety, quality, and efficacy within their jurisdictions. These differences can present challenges for multinational companies operating in the food manufacturing sector, necessitating a nuanced approach to compliance.
Strategies for managing multinational cGMP compliance effectively include establishing a centralized quality management system incorporating the most stringent aspects of all relevant regional regulations. To assist in this initiative, we have a comprehensive list of the best QMS solutions that can be invaluable. It’s because leveraging technology for compliance management can provide global visibility and control over quality processes, facilitating more accessible adaptation to changes in regulatory landscapes. Finally, continuous employee training and development across all locations emphasize a culture of quality and compliance, ensuring that standards are uniformly understood and applied, thereby minimizing non-compliance risks.
What is the purpose of inspections and audits in Ensuring cGMP Compliance?
Regular internal audits and external inspections serve as critical checkpoints in the cGMP compliance process. These evaluations help identify non-conformities and opportunities for improvement, which are necessary for maintaining compliance.
The key to successful audits and inspections is preparation:
- Conduct regular self-audits to identify and address compliance issues
- Keep detailed and up-to-date records that can be easily accessed during inspections
- Implement corrective action plans based on audit findings
Businesses can strengthen their cGMP compliance endeavors by reframing audits and inspections as chances for enhancement rather than obstacles. Consistent audits and inspections ensure compliance and foster a culture of excellence and continuous improvement within the organization.
How to Get cGMP Certification?
Achieving Current Good Manufacturing Practices (cGMP) certification is essential for food manufacturers, dietary supplement producers, and other industries seeking compliance with FDA regulations and global safety standards.
cGMP certification ensures that products are consistently manufactured and controlled according to stringent quality standards, minimizing risks of contamination, errors, and defects.
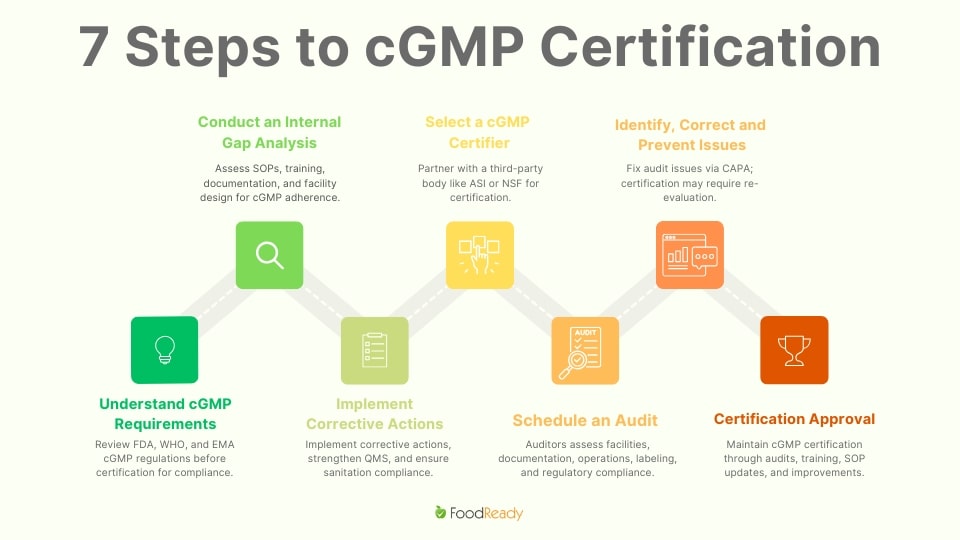
Below is a step-by-step guide on how to obtain cGMP certification:
Step 1: Understand cGMP Requirements
Before starting the certification process, it is crucial to thoroughly review cGMP guidelines relevant to your industry. These regulations are outlined by the FDA (21 CFR Part 110, 111, 117) and other global agencies like the WHO and EMA. cGMP compliance covers various aspects, including:
- Facility design and maintenance
- Equipment calibration and validation
- Staff training and hygiene protocols
- Documentation and record-keeping
- Quality control and product testing
- Proper storage and distribution
Step 2: Conduct an Internal Gap Analysis
Evaluate your current manufacturing processes against cGMP standards by conducting an internal audit. This step helps identify areas that require improvement, such as:
- Lack of updated Standard Operating Procedures (SOPs)
- Inadequate employee training programs
- Poor documentation or traceability
- Facility design flaws that may lead to contamination
Step 3: Implement Corrective Actions
Based on the internal audit, take necessary actions to address gaps and improve compliance. This may involve:
- Updating SOPs to reflect cGMP guidelines
- Implementing a Quality Management System (QMS)
- Enhancing employee training on food safety and quality control
- Establishing supplier and material verification processes
- Ensuring cleaning and sanitation protocols are in place
Step 4: Choose a cGMP Certification Body
To obtain certification, you need to work with a third-party certification body that conducts audits and verifies compliance. Some of the leading cGMP certifying bodies include:
- ASI Food Safety
- NSF International
- SGS
- Eurofins
- UL Solutions
Certification costs vary based on facility size, number of locations, number of employees, and complexity of operations. The audit price can range from a few thousand to tens of thousands of dollars, depending on scope.
Step 5: Schedule a cGMP Audit
The certification body will schedule an on-site cGMP audit where an auditor will review:
- Facility Conditions – Cleanliness, layout, and equipment maintenance
- Documentation – SOPs, batch records, employee training logs, CAPAs
- Operational Processes – Manufacturing, quality control, and packaging
- Product Testing & Labeling – Ensuring product consistency and compliance
- Regulatory Adherence – Compliance with FDA and industry-specific guidelines
Step 6: Address Non-Conformities & Submit Corrective Actions
If any non-conformities are identified during the audit, you must implement Corrective and Preventive Actions (CAPA) within a specified timeframe. A follow-up audit may be required to confirm compliance.
Step 7: Certification Approval & Ongoing Compliance
Once all requirements are met, the certification body issues a cGMP certificate. However, cGMP compliance is not a one-time achievement—it requires ongoing monitoring and periodic re-audits to maintain certification.
Best practices to maintain cGMP compliance include:
- Conducting regular internal audits
- Keeping employee training programs updated
- Reviewing and revising SOPs annually
- Implementing continuous process improvements
- Staying updated on regulatory changes
Finally, if you’re looking to get cGMP certified, we offer tailored support to help your business meet regulatory standards and pass certification audits.
Advancements and Trends in cGMP
The Current Good Manufacturing Practices landscape is continually evolving, driven by technological innovations and shifts in manufacturing practices. These advancements enhance efficiency, compliance, and product quality in the food manufacturing sector.
Technological Innovations Impacting cGMP
Modern technology plays a pivotal role in transforming cGMP compliance. Digitalization, through the adoption of Industry 4.0 technologies such as the Internet of Things (IoT), Artificial Intelligence (AI), and blockchain, enables more precise monitoring and control of manufacturing processes.
IoT devices facilitate real-time tracking of environmental conditions and process parameters, while AI algorithms can predict and prevent deviations before they occur. Blockchain offers unprecedented traceability in supply chains, ensuring the authenticity and safety of raw materials and finished products.
Future Directions in Manufacturing Practices
Looking ahead, the adoption of personalized nutrition and on-demand production is leading to smaller, more flexible manufacturing setups. This shift requires a reevaluation of traditional cGMP standards to address the challenges of scalability and customization.
Additionally, the industry is moving towards more sustainable manufacturing practices, focusing on reducing waste and energy consumption. These ecological considerations are becoming integral to cGMP compliance, reflecting a broader commitment to environmental responsibility alongside consumer safety.
Summary
cGMP is the linchpin of modern food manufacturing, ensuring that the products consumers rely on are produced in a safe, consistent, and reliable manner. Achieving and maintaining cGMP compliance is no small feat, but the benefits of consumer safety, industry reputation, and business success are substantial.
If you understand the importance of cGMP and take a proactive approach to compliance and continuously improve your processes, you can set your business apart as a leader in food safety and quality. From detailed documentation to rigorous training and thorough inspections, each component of cGMP underscores your commitment to excellence.
In the food industry, cGMP is not merely a set of guidelines to follow, but a promise to consumers that the products they bring into their homes are good, safe, and faithful to their labels. Through diligence and dedication, the principles of cGMP can be woven into the very fabric of your organization, creating a reliable framework for success.
FAQs
cGMP stands for Current Good Manufacturing Practices. The FDA enforces these regulations to ensure the safety and efficacy of food products, pharmaceuticals, and medical devices.
The lowercase “c” in cGMP stands for “current,” emphasizing that Good Manufacturing Practices (GMP) must continuously evolve to reflect the latest technologies, regulations, and industry standards.
cGMP regulations ensure that products are consistently produced and controlled according to quality standards, minimizing the risks involved in food manufacturing, pharmaceutical production, and the creation of medical devices. This helps protect consumers from products that do not meet safety, quality, or efficacy standards.
The frequency of updates to cGMP regulations varies, depending on technological advancements, emerging industry practices, and lessons learned from past compliance issues. The FDA and other regulatory bodies continuously monitor and assess the need for updates to ensure the guidelines remain relevant and effective.
Non-compliance with cGMP regulations can lead to severe consequences, including product recalls, fines, and legal action. Additionally, it can harm a company’s reputation, losing consumer trust and business opportunities. It is essential to maintain compliance to avoid these consequences and ensure the safety and efficacy of products.
To become cGMP compliant, a company should first conduct a thorough review of the cGMP guidelines relevant to its industry. This involves understanding the specific requirements for documentation, quality control, personnel qualifications, and facility conditions. Following this, companies should assess their current operations against these standards and develop a comprehensive plan to address gaps. Training employees on cGMP principles and practices, establishing a quality management system, and implementing consistent documentation and record-keeping procedures are critical.
While implementing cGMP standards can present financial challenges for small businesses, the long-term benefits usually outweigh the initial costs. Small businesses can approach cGMP implementation in a phased manner, prioritizing critical areas that directly impact product quality and safety. Many regulatory bodies offer guidance and resources tailored to assist small businesses in achieving compliance. Furthermore, demonstrating adherence to cGMP standards can open new market opportunities and enhance customer trust, which is invaluable for business growth.
While third-party certification is not a mandatory requirement for cGMP compliance, obtaining such certification can provide additional credibility and assurance to customers and regulatory bodies. Third-party certifiers conduct independent audits to verify that a company’s manufacturing processes meet cGMP standards. This certification can be a powerful marketing tool, showcasing a company’s commitment to maintaining high-quality and safe manufacturing practices







