Has a business such as Whole Foods, Costco, Publix, Kroger, Trader Joe’s or Aldi told you that you need a cGMP aka GMP certification (Good Manufacturing Practices) before they will sell your product in their stores?
A food production business can hire an independent firm to perform a GMP audit to certify they are operating under CGMP standards and are producing safe food. A GMP audit is required by many retailers and can help you get your product into many stores. GMP certification requires you to have a HACCP plan as well as other requirements which I will go into detail later.
After you read the requirements, you might decide you need help! FoodReady software and consulting can help get your facility, team, and process “audit ready” for a GMP audit. It’s the easy route to get GMP-certified.
GMP Compliance vs Certification
It’s important to distinguish between GMP compliance and GMP certification. GMP compliance means your facility follows FDA-mandated Current Good Manufacturing Practices (cGMP) as outlined in 21 CFR Part 117, often assessed through regulatory inspections.
GMP certification, on the other hand, is a voluntary process in which a third-party audits your facility and formally verifies that it meets GMP standards. While compliance is required by law, certification serves as additional proof of your commitment to safety and quality, often requested by retailers or international partners.
What Are the Requirements of a GMP Certification?
1. Facility Requirements
The plant and grounds must be in sanitary working order according to CGMP specifications. The plumbing must be in sanitary and working order according to CGMP guidelines.
2. Employees
Employees must not be sick and must follow hygiene practices so as not to contaminate food products.
3. Sanitary Operations
You can only use safe non-toxic cleaning chemicals around food production. Toxic chemicals are only allowed if absolutely necessary in the cleaning of equipment or other instances where only that specific chemical can be used. Any toxic chemicals must be stored away from ingredients and food production in a secure manner.
Pests must be controlled, and pesticides can only be used when it will not result in food contamination.
You must clean any surface that comes in contact with food.
Dry food areas must be kept clean and dry.
Wet food processing areas must be sanitized before and after use.
Single-use items must be protected from contaminating elements.
4. Equipment and Utensils
Equipment and utensils must be cleanable and in clean condition and cannot shed metal fragments or lubricants.
Thermometers must be in all cooling and freezing machinery and be in working order.
PH and measuring devices must be accurate.
Compressed air must be used in a way so as not to contaminate food.
5. Processes and Controls
All parts of the processing operation must have proper sanitation.
Trained employees must be responsible for proper cleaning.
Food must be protected from contamination and allergens.
Testing must be performed to identify if there are any areas that have not been sanitized properly (such as swabs sent to labs, or ATP testing).
Contaminated food must be disposed of properly.
Packaging must be safe.
6. Raw Materials and Ingredient
Ingredients must be kept at proper temperatures, in safe containers away from contaminants, pests and allergens, they must be safe for ingestion, ingredients should be inspected for cleanliness, and if susceptible to toxins, made sure that they are toxin free.
7. Manufacturing Operations
Equipment and utensils are to be kept clean and maintained and used and constructed in a way that does not contaminate food.
Refrigerated food must be continually refrigerated.
When food is not protected, it must be carefully handled so contamination does not occur.
The food production process should be constructed to eliminate bacterial growth and contamination.
Food should be protected from foreign objects such as metal shards.
Contaminated food must be disposed of or “reworked”. Any food that is reworked, contamination, and bacterial growth must be eliminated.
Dry food must be kept dry.
Only food-quality ice should be used.
Foods that rely on acidification for safety must be (pH≤4.6).
Foods that are open are used repeatedly must be kept safe from contamination. Examples would be dips and coatings.
The packaging and filling and assembling processes must not introduce contaminates.
8. Warehousing and Distribution
Food storage buildings and distribution vehicles must protect the food from contaminants.
9. Holding and Distribution of Human Food By-Products for use in Animal Feed
By-products must be held in safe containers, labeled using common names, and protected from any contamination.
Shipping containers must be inspected and not contaminated before use.
10. Defect Action Levels
Consistent quality control must be employed to detect defects.
Contaminated and uncontaminated food must never be mixed.

The 5 Ps of GMP
The 5 Ps of GMP offer a simple, memorable way to understand the core elements of a GMP-compliant facility:
- People: Employees must be trained, qualified, and committed to following hygiene and safety protocols. Their behavior directly impacts product quality.
- Premises: Facilities should be designed to prevent contamination, with proper workflow, ventilation, and sanitary infrastructure.
- Processes: Standard Operating Procedures (SOPs) should govern all production activities, ensuring consistency and traceability.
- Products: Ingredients and finished goods must be stored, handled, and tested appropriately to maintain safety and integrity.
- Procedures: Detailed documentation is critical to demonstrate compliance, enable audits, and support corrective actions when issues arise.
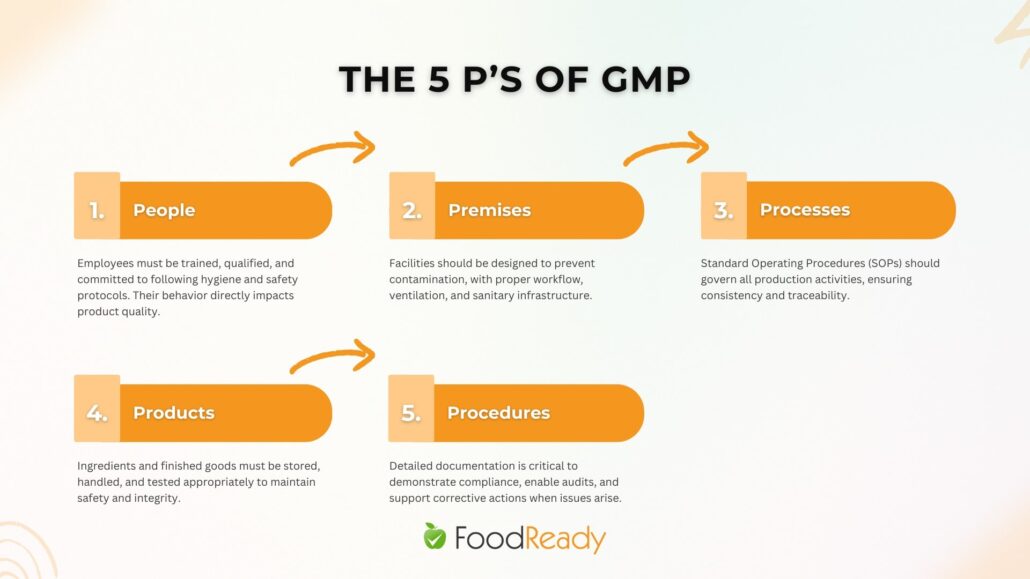
These five pillars support a strong food safety foundation, guiding daily operations toward consistent quality and regulatory readiness.
6-Step GMP Compliance Checklist
Getting GMP certified doesn’t need to be overwhelming. Use this simple checklist to guide your compliance journey:
1. Review the CGMP Standards
Start familiarizing yourself with the FDA’s Current Good Manufacturing Practices (21 CFR Part 117 for food). This sets the foundation for every requirement you’ll need to meet.
2. Conduct a Facility Gap Assessment
Walk through your facility and document any areas that don’t align with GMP requirements, such as hygiene practices, pest control, equipment condition, or employee training.
3. Develop or Update Your Food Safety Plan
A GMP-compliant operation requires a written food safety plan. This includes a HACCP plan, sanitation procedures, SOPs, and monitoring protocols.
4. Train Employees on GMP Protocols
Every staff member, from line workers to supervisors, must understand how to prevent contamination, follow hygiene rules, and respond to food safety risks.
5. Implement Corrective Actions and Records
Address any deficiencies identified in your gap analysis, and set up proper recordkeeping systems for traceability, cleaning, temperature checks, and quality control.
6. Schedule Your GMP Audit
Once you’re confident in your systems, contact an accredited third-party auditing body to perform a GMP audit and issue your certification.
Pass Your Audit and Get GMP Certification
With FoodReady’s guided prep, automated monitoring, and expert support, certification is within reach.
How Does FoodReady Help With GMP Tools And Software?
| Tool Type | Purpose in GMP Compliance | How FoodReady Helps |
|---|---|---|
| HACCP Plan Builder | Designs hazard control plans and CCPs | Drag-and-drop builder tailored to GMP-aligned workflows |
| Digital Checklists & Logs | Ensures sanitation, temperature, and process documentation | Customizable checklists with mobile access and cloud backups |
| Document Control System | Maintains SOPs, training records, and revision tracking | Auto-versioning, searchable document library |
| Corrective Action Management | Tracks non-conformances and resolutions | Built-in CAPA workflows with alerts and follow-ups |
| Audit Management | Prepares for internal and third-party audits | Audit scheduling, readiness dashboards, and compliance scoring |
| Traceability & Inventory | Tracks ingredients from receiving to distribution | Full lot traceability, recalls, and expiration tracking |
Want personalized guidance on GMP software or audit prep?
Talk to a FoodReady consultant and get expert support tailored to your facility’s needs.
Summary
As you can see, preparing for a GMP audit is a daunting task. Employing FoodReady and our food safety software, AI HACCP builder, and/or one of our food safety consultants can substantially lessen your quality assurance workload. Many businesses use FoodReady software and consulting as a substitute for a full-time QA manager.
FoodReady is a food safety software and consulting company. Our software has a HACCP plan builder, food traceability, you can create your own checklists to manage your HACCP plan or track other items, ingredients, tasks, or events. With our Enterprise program, you will have access to food safety quality assurance professionals who can help you become GMP-certified, as well as assist with GFSI, SQF, GMP, SOP, BRC, CGMP, HACCP, gap analysis, or prepare for audits like the Costco audit, the Whole Foods audit, Publix audit, Kroger audit, Safeway audit, Meier audit, HEB audit and more.
FAQs
Common reasons include inadequate documentation, poor sanitation and hygiene practices, improper storage of materials, lack of proper training for employees, and insufficient quality control measures.
In many industries, especially in food, pharmaceuticals, and cosmetics, GMP certification is a regulatory requirement. It ensures that products are safe, of high quality, and produced under controlled conditions. However, the requirement can vary by country and specific product category.
The time frame for GMP certification can vary depending on the size and complexity of the facility, the current level of compliance, and the readiness of the facility for the audit. Generally, it can take several months to prepare for the audit, and once the audit is successfully completed, certification can be issued within a few weeks.







