When you find out that food you’ve bought or have in stock has been recalled, you need to act fast. Every year, millions of food products get recalled across the US. Everything from contaminated items with nasty bacteria to stuff with hidden allergens or weird things like glass shards and metal pieces. Recalls can also involve consumer products that pose risks to the food supply. Serious outcomes, including illness and even death, can happen if recalled food is not handled properly.
Facing a recall situation regulated by the Food and Drug Administration (FDA) can be a daunting and challenging experience for food manufacturers, retailers, and distributors. An FDA recall is initiated when a product is deemed unsafe, defective, or violative of regulations, posing a risk to public health. Understanding the intricate process of handling an FDA recall is crucial for mitigating potential risks, protecting consumers, and safeguarding brand reputation.
This comprehensive guide explores the key steps, best practices, and strategic approaches to manage and navigate an FDA recall crisis effectively.
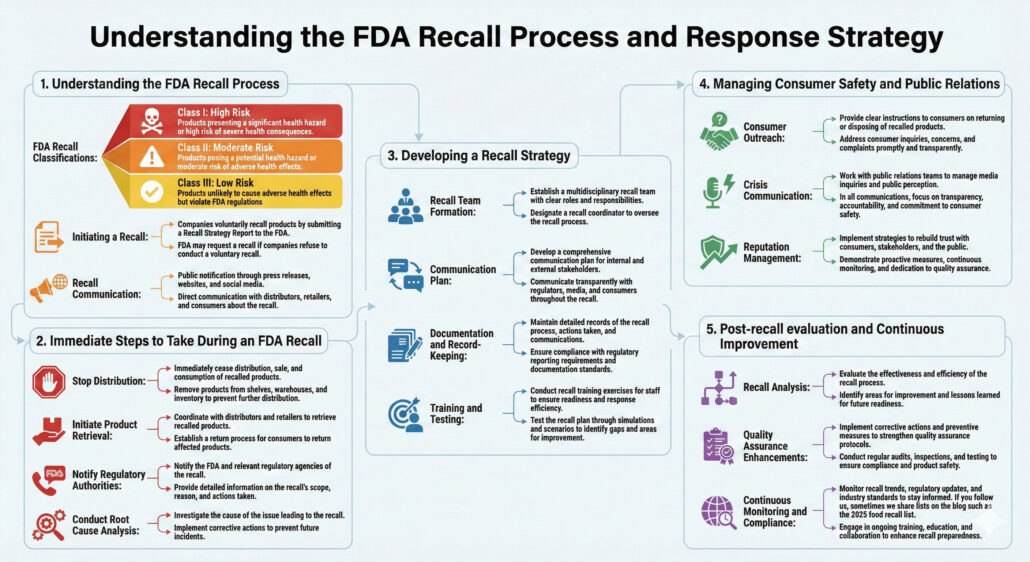
1. Understanding the FDA Recall Process
FDA Recall Classifications:
- Class I: Products presenting a significant health hazard or high risk of severe health consequences.
- Class II: Products posing a potential health hazard or moderate risk of adverse health effects.
- Class III: Products unlikely to cause adverse health effects but violate FDA regulations
Initiating a Recall:
- Companies voluntarily recall products by submitting a Recall Strategy Report to the FDA.
- FDA may request a recall if companies refuse to conduct a voluntary recall.
Recall Communication:
- Public notification through press releases, websites, and social media.
- Direct communication with distributors, retailers, and consumers about the recall.
2. Immediate Steps to Take During an FDA Recall
Stop Distribution:
- Immediately cease distribution, sale, and consumption of recalled products.
- Remove products from shelves, warehouses, and inventory to prevent further distribution.
Initiate Product Retrieval:
- Coordinate with distributors and retailers to retrieve recalled products.
- Establish a return process for consumers to return affected products.
Notify Regulatory Authorities:
- Notify the FDA and relevant regulatory agencies of the recall.
- Provide detailed information on the recall’s scope, reason, and actions taken.
Conduct Root Cause Analysis:
- Investigate the cause of the issue leading to the recall.
- Implement corrective actions to prevent future incidents.
3. Developing a Recall Strategy
Recall Team Formation:
- Establish a multidisciplinary recall team with clear roles and responsibilities.
- Designate a recall coordinator to oversee the recall process.
Communication Plan:
- Develop a comprehensive communication plan for internal and external stakeholders.
- Communicate transparently with regulators, media, and consumers throughout the recall.
Documentation and Record-Keeping:
- Maintain detailed records of the recall process, actions taken, and communications.
- Ensure compliance with regulatory reporting requirements and documentation standards.
Training and Testing:
- Conduct recall training exercises for staff to ensure readiness and response efficiency.
- Test the recall plan through simulations and scenarios to identify gaps and areas for improvement.
4. Managing Consumer Safety and Public Relations
Consumer Outreach:
- Provide clear instructions to consumers on returning or disposing of recalled products.
- Address consumer inquiries, concerns, and complaints promptly and transparently.
Crisis Communication:
- Work with public relations teams to manage media inquiries and public perception.
- In all communications, focus on transparency, accountability, and commitment to consumer safety.
Reputation Management:
- Implement strategies to rebuild trust with consumers, stakeholders, and the public.
- Demonstrate proactive measures, continuous monitoring, and dedication to quality assurance.
5. Post-recall evaluation and Continuous Improvement
Recall Analysis:
- Evaluate the effectiveness and efficiency of the recall process.
- Identify areas for improvement and lessons learned for future readiness.
Quality Assurance Enhancements:
- Implement corrective actions and preventive measures to strengthen quality assurance protocols.
- Conduct regular audits, inspections, and testing to ensure compliance and product safety.
Continuous Monitoring and Compliance:
- Monitor recall trends, regulatory updates, and industry standards to stay informed. If you follow us, sometimes we share lists on the blog such as the 2025 food recall list.
- Engage in ongoing training, education, and collaboration to enhance recall preparedness.
How FoodReady Software Can Assist with FDA Recalls
FoodReady is a software that facilitates recall management, helping food and beverage companies efficiently handle FDA recalls and ensure compliance.
Track Lots in Real Time and Stay FSMA 204 Compliant
With FoodReady, you can trace affected lots in minutes using real-time tracking and FSMA 204-ready records. Instead of digging through spreadsheets or paper files, you’ll know right away which customers got the recalled product, how much went where, and when.
Manage and Document Recalls
The platform’s recall tools let you:
- Create standard recall notices
- Document every decision and action
- Keep a complete recall log for audits and inspections
- Track how well you’re doing and who you’ve notified
This documentation protects you legally and shows auditors you’re following the right procedures.
Practice with Mock Recalls
You can run recall simulations and mock recalls in FoodReady AI to test if your team’s ready. These practice runs help find gaps in your SOPs, improve your HACCP plans for recall scenarios, and make sure everyone knows their job before a real recall hits.
Track Suppliers and Ingredients
The software helps manage supplier verification and ingredient tracking so you can quickly see if a supplier recall affects your finished products. It traces the impact across your whole supply chain and figures out exactly how far contamination might’ve spread through your inventory.
Get Expert Help
FoodReady AI’s consulting team helps build or update recall SOPs, product hold procedures, and communication templates. They make sure your protocols match up with SQF, BRCGS, and other certifications your customers need.
Are you prepared to act quickly when a food recall hits?
Learn how to protect consumers, stay compliant, and manage recalls confidently with clear steps and the right tools in place.
Why Choose FoodReady for FDA Recalls:
- Industry-Specific Solution:
- Tailored specifically for the food and beverage industry, addressing unique regulatory challenges and requirements.
- User-Friendly Interface:
- Intuitive and easy to use, ensuring quick adoption by your team with minimal training.
- Food Recall Specialists:
- Food recall consultant helping food and beverage companies efficiently handle recalls and ensure compliance.
Conclusion
Navigating an FDA recall requires meticulous planning, swift action, clear communication, and unwavering commitment to consumer safety and regulatory compliance. By following the outlined steps, developing a comprehensive recall strategy, and prioritizing transparency and accountability, organizations can effectively manage an FDA recall crisis, minimize risks, and protect public health. Handling an FDA recall with integrity, professionalism, and dedication to quality is a regulatory obligation and a moral imperative to prioritize consumer welfare, uphold industry standards, and safeguard brand reputation in the face of adversity.
FAQ
An FDA recall is an action taken to remove a product from the market that violates FDA laws. Recalls are initiated when a product is defective or potentially harmful.
There are three classes of FDA recalls:
–Class I: Products that could cause serious health problems or death.
–Class II: Products that might cause a temporary health problem or pose a slight threat of a serious nature.
–Class III: Products unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws.
If necessary, the manufacturer, distributor, or the FDA can initiate a recall to protect public health.
– Identify the Issue: Determine the scope and nature of the problem.
– Notify the FDA: Inform the FDA about the issue and your recall plan.
– Initiate Recall: Communicate with distributors, retailers, and consumers about the recall.
– Retrieve the Product: Remove the product from the market and manage its return or destruction.
– Corrective Actions: Implement measures to prevent future occurrences.
You must submit a report to the FDA, including details about the product, the issue, and your proposed recall strategy. You can do this through the FDA’s electronic recall submission process or by contacting the appropriate FDA district office.
A recall notification should include the product name, description, lot or serial numbers, reasons for the recall, health hazard assessment, instructions for handling the recalled product, and contact information for further inquiries.
Use channels such as press releases, social media, direct notifications, and your website to inform customers and the public about the recall. Provide clear instructions on what they should do if they have the recalled product.
According to FDA guidelines, recalled products should be returned to the manufacturer or designated location, quarantined, repaired, reconditioned, or destroyed.
Monitor the recall process closely, track the return and disposition of the recalled product, and conduct effectiveness checks to ensure all affected products are accounted for and removed from the market.
Evaluate the recall process, implement corrective actions to address the root cause of the problem, and prepare a final report to the FDA documenting the recall process and outcomes.
Enhance your quality control processes, conduct regular audits, ensure compliance with all regulatory requirements, and provide thorough training for your staff on quality and safety standards.
For more detailed information, you can visit:
Industry Guidance For Recalls
FDA: Recalls, Market Withdrawals, & Safety Alerts
FDA: Guidance for Industry – Product Recalls, Including Removals and Corrections







