Achieving HACCP compliance is vital for regulatory adherence, ensuring food safety, and maintaining access to key markets. This guide is designed for food manufacturers, processors, co-packers, and distributors aiming to meet HACCP requirements through modern traceability software.
Food traceability software represents the most efficient method to fulfil HACCP requirements in 2026. HACCP (Hazard Analysis and Critical Control Points) became the global benchmark for preventive food safety after its development by NASA and Pillsbury in the 1960s and was formalized by Codex Alimentarius in 1993. Traditionally, food manufacturers relied on paper logs and manual processes to document their HACCP systems. While this worked in simpler supply chains with less stringent regulatory demands, today’s complex food environments require more robust solutions.
Food traceability is the capability to track food products and their ingredients throughout every stage of production, processing, and distribution. It is a cornerstone of the HACCP approach, enabling the monitoring and identification of hazards in the supply chain and supporting a comprehensive quality management system. A critical element of HACCP is ensuring that products can be traced, which is essential for managing food safety risks effectively.
The regulatory landscape shifted significantly with the Food Safety Modernization Act (FSMA) of 2011 and the FSMA 204 food traceability final rule finalized in 2022. Regulators now expect food businesses to demonstrate that their HACCP plans are not just documented but actively effective. The 2018 romaine lettuce E. coli outbreak, which affected over 200 people across 36 states, highlighted how incomplete traceability delayed identifying the contamination source by weeks. Similarly, the 2023 frozen strawberry hepatitis A outbreak, involving multiple suppliers, showed that even well-designed HACCP programs can fail without precise traceability to determine affected lots.
For manufacturers, processors, co-packers, and distributors, the message is clear: HACCP is no longer just a compliance document for audits. It is a living system that depends on real-time data about raw materials, production processes, and finished goods. Food traceability software captures this data, enabling rapid responses when critical control point deviations occur or suppliers report potential contamination. In such cases, answers are needed within hours.
FoodReady offers an all-in-one, AI-powered food safety and traceability platform that helps teams build HACCP plans and operationalize them through digital records, lot tracking, automated monitoring, and recall simulation. This platform bridges the gap between having a HACCP plan and proving its effectiveness every day.
HACCP Fundamentals: Integrating Traceability Within the Seven Principles
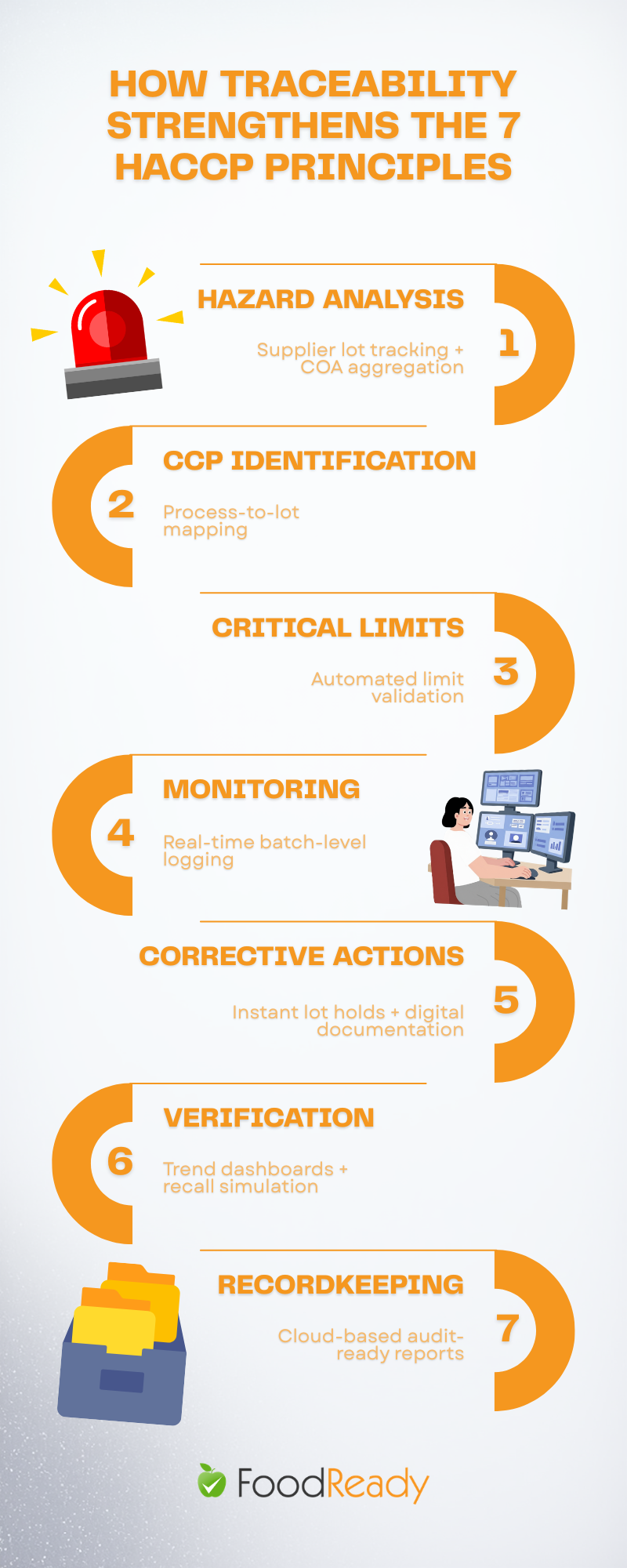
The seven Codex HACCP principles form the foundation for controlling food safety hazards in any production process. Food traceability software primarily strengthens Principles 1 through 6, ranging from hazard analysis to verification, while significantly enhancing Principle 7, which involves documentation and record-keeping.
Each principle requires specific data that traceability systems address:
- Principle 1 (Conduct Hazard Analysis): Requires understanding potential biological, chemical, and physical hazards linked to ingredients and processes. This involves supplier history, certificates of analysis, and ingredient origin tracking.
- Principle 2 (Determine Critical Control Points): Identifies where controls must be applied, necessitating mapping CCPs to production lines and product types using process flow data.
- Principle 3 (Establish Critical Limits): Defines measurable thresholds at each CCP, linked to specific products and consistently monitored.
- Principle 4 (Establish Monitoring Procedures): Requires ongoing data collection, such as temperatures, times, pH levels, and metal detector checks, tied to specific batches.
- Principle 5 (Establish Corrective Actions): Demands documentation of incidents, corrective steps, and product disposition when limits are exceeded.
- Principle 6 (Verification): Needs trend data and historical records to prove the system’s ongoing effectiveness.
Traceability data, including supplier lots, production batches, process parameters, and distribution records, provides the evidence auditors and regulators use to evaluate HACCP effectiveness. Without connected and accessible data, even the best-written HACCP plan remains theoretical.
The following sections detail how food traceability software like FoodReady reinforces each HACCP principle in daily operations.
From Hazard Analysis to Lot-Level Control: How Traceability Software Supports HACCP Steps
Transitioning from HACCP documentation to operational reality requires linking each principle to actual production data. Understanding food traceability and its regulatory drivers clarifies why this connection is essential across today’s complex supply chains. Here’s how traceability software enhances each step: :
Supplier Data Aggregation
During hazard analysis, digital traceability platforms compile supplier performance scores, certificates of analysis, historical deviations, and complaint data by ingredient lot, often leveraging key technologies in food traceability. This streamlines identifying high-risk foods, such as leafy greens, soft cheeses, ready-to-eat deli meats, and fresh fruits, that require extra controls. Instead of sifting through paper files, QA teams can filter data instantly by vendor, ingredient category, or risk rating.
CCP Validation
Lot and process tracking link control measures to specific products. For example, associating oven temperature logs and cook times with ground beef lots verifies E. coli O157:H7 control. Pasteurization records tied to milk batches confirm Listeria control. This lot-level linkage transforms generic CCP documentation into verifiable evidence.
Real-Time Monitoring
Real-time production tracking connects CCP monitoring entries, metal detector checks, pH measurements, chlorine levels, to exact batches, lines, and shifts. Every record includes timestamps and user IDs, creating complete digital trails that satisfy rigorous auditor scrutiny.
Deviation Response
When a CCP deviation occurs, such as an undercook or allergen control failure, the traceability system can automatically hold affected lots, prompt corrective workflows, and document product disposition. This ensures non-conforming products do not ship and creates records required for verification.
Verification Activities
Verification and validation tasks, such as internal audits, environmental swabs, and calibration records, can be tagged to specific locations, lines, and products. This proves the HACCP plan functions as intended and corrective actions are effective over time.
FoodReady’s HACCP plan builder uses templates aligned with Codex, SQF, and BRCGS standards and integrates with traceability, monitoring, and corrective action modules, similar to other leading food traceability software platforms. This keeps plans and operations synchronized, preventing drift.
Digitizing HACCP Records: From Paper Logs to Real-Time Traceability
Traditional paper HACCP logs, clipboards, handwritten sheets, binders, have served their purpose but come with limitations: transcription errors, illegible handwriting, missing entries, and time-consuming audit preparation, which modern HACCP software solutions are designed to overcome.
Advantages of Digital Recordkeeping
Centralized, cloud-based records transform HACCP management. Records become searchable and filterable by lot, date, line, and product:
| Record Type | Paper Challenges | Digital Benefits |
|---|---|---|
| Receiving logs | Lost documents, incomplete data | Scanned COAs linked automatically to lot codes |
| CCP monitoring | Missing entries, unclear handwriting | Time-stamped, validated against limits |
| Sanitation SSOPs | Difficult verification | Photo documentation and completion tracking |
| Allergen changeovers | Inconsistent documentation | Checklists with required fields and signatures |
| Calibration records | Filed separately | Linked to equipment and monitoring data |
| Food traceability software captures records via mobile devices, tablets, or barcode scanners at the point of activity, often integrating closely with food inventory management software. Time-stamped entries with user IDs create accountability. Automatic reminders prompt timely checks, while validation rules prevent out-of-spec entries from going unnoticed. |
Audit Preparation Benefits
Digital records dramatically reduce audit preparation time. QA teams can generate HACCP record packets for specific SKUs, dates, or production runs in minutes, critical during unannounced audits.
FoodReady integrates HACCP forms, SOPs, and SSOPs with traceability data, presenting a single batch record that includes ingredients, CCP checks, sanitation status, and release decisions, exactly what auditors require.
FSMA 204, HACCP, and Traceability: Aligning Regulatory Expectations
FSMA 204 builds on FSMA’s preventive approach by requiring businesses on the Food Traceability List to capture standardized traceability data, including receiving, transformation, and shipping records, enabling regulators to trace lots quickly, often within 24 hours. This FSMA 204 food traceability rule strengthens HACCP verification and audit readiness.
For a detailed breakdown of FSMA 204 requirements and FoodReady support, see our full guide.
Real-Time Traceability as Proof of HACCP Corrective Action and Verification
Certain HACCP hazards, such as allergens, biological contamination, and foreign material, require immediate, documented responses. Without real-time traceability, corrective actions cannot be executed precisely, verified effectively, or defended during audits. Modern traceability systems provide the operational backbone, transforming HACCP from static procedures into active control.
Allergen Control Applications (Principles 5 & 7)
Allergen cross-contact is a high-severity hazard needing clear corrective actions. When a mislabeled whey powder lot is identified, traceability software enables teams to isolate affected SKUs, validate sanitation logs, and document holds or recalls at the lot level.
This supports:
- Principle 5 (Corrective Actions): Immediate containment and documented disposition.
- Principle 7 (Documentation): Time-stamped records detailing what occurred, who responded, and how the hazard was controlled.
This precision reduces recall scope, targeting only impacted lots while maintaining HACCP defensibility.
CCP Deviation Response (Principles 5 & 6)
For CCP deviations, such as undercooking or environmental Listeria positives, real-time lot tracking allows QA teams to hold specific pallets, runs, or line segments. Inventory visibility shows product location, whether onsite, in transit, or at customers.
This supports:
- Principle 5 (Corrective Actions): Immediate segregation and disposition.
- Principle 6 (Verification): Evidence that corrective measures were effective and contained.
Without traceability integration, proving control over the affected product is nearly impossible.
Recall Simulation as HACCP Verification (Principles 6 & 7)
Recall capability is more than crisis management; it is structured proof that HACCP functions correctly. Automated recall simulations test whether traceability data, monitoring records, and corrective actions connect seamlessly.
Mock recalls support:
- Principle 6 (Verification): Demonstrating system performance under stress.
- Principle 7 (Documentation): Generating complete, auditable reports including lot codes, quantities, customer locations, ship dates, and dispositions.
Proactive mock recalls identify gaps before regulatory findings or public health incidents occur.
Building a HACCP-Compliant Traceability System with FoodReady
FoodReady is not just a traceability add-on; it is the infrastructure making HACCP executable, measurable, and defensible daily. Instead of managing hazard analysis, monitoring, records, and lot tracking in disconnected systems, FoodReady connects them into a unified operational framework where every CCP, corrective action, and verification links directly to production data, similar in spirit to a dedicated HACCP plan builder for safety plans.
HACCP Plan Builder: From Static Document to Live System
A HACCP plan gains meaning only when hazards, CCPs, and critical limits connect to operational records. FoodReady’s plan builder translates Codex-aligned hazard analysis into digital monitoring forms, corrective workflows, and traceability links.
AI-assisted hazard analysis identifies biological, chemical, and physical hazards based on ingredients and processes. Critical Control Points in the HACCP plan are defined and tied directly to monitoring records, lot codes, and product flows, ensuring auditors see both the plan and batch-level evidence.
Verification requires this connection; without it, validation is theoretical.
SOP and SSOP Integration: Supporting Prerequisite Programs
Prerequisite programs such as GMPs and SSOPs underpin HACCP. FoodReady integrates SOPs and sanitation procedures with monitoring and traceability data.
Sanitation logs, allergen changeover records, and training data link directly to production lots and CCPs, strengthening Principle 6 (Verification) by showing prerequisite controls function as operational safeguards.
Lot-Level Traceability as HACCP Evidence
Verification requires linking CCP monitoring records to lot codes. FoodReady uses GS1-compatible identifiers and digital scanning to connect ingredient lots, production batches, and finished goods. Every receiving, transformation, and shipping event ties to CCP data, creating verifiable backward and forward traceability, direct evidence for corrective actions, and audit-ready documentation aligned with FSMA 204 and GFSI standards.
Traceability is the data backbone of HACCP, not a separate function.
Intelligent Monitoring as Preventive Enforcement
HACCP depends on consistent monitoring and immediate response. FoodReady’s rules engine flags missing CCP checks, expired ingredients, temperature deviations, and incomplete corrective actions in real time.
Instead of discovering gaps during audits, teams receive alerts when controls drift, strengthening Principles 4 and 5 and ensuring continuous system operation.
Consulting Support: Aligning Technology with Real Hazards
Technology alone does not guarantee HACCP success. FoodReady’s experts help validate CCPs, refine hazard analysis, configure workflows, and align digital forms with regulations and certification schemes.
Combining software and expertise ensures the traceability system reflects actual risks, accelerates compliance, and maintains technical integrity.
Ready to strengthen your HACCP system with real-time traceability?
Book a FoodReady demo today.
Implementation Roadmap: Transitioning from Paper HACCP to Digital Traceability
Many facilities still rely partially on paper in 2025-2026, with HACCP logs in binders and traceability data scattered across spreadsheets and systems. A phased rollout reduces risk and resistance while delivering compliance benefits and improved supply chain management quickly, especially when paired with a structured approach to creating and implementing a HACCP plan.
Step-by-Step Implementation
- Current-State Assessment: Evaluate existing HACCP plans, traceability gaps, and record-keeping. Identify delays and errors in paper processes. Map data flows from receiving to shipping.
- Product and Process Flow Mapping: Document ingredient sources, processing steps, CCPs, packaging, and distribution channels to configure traceability solutions.
- System Configuration: Set up HACCP plans, monitoring forms, and traceability parameters in FoodReady AI, aligning templates with existing plans.
- Pilot Implementation: Begin with 1-2 lines or facilities handling high-risk or FSMA 204 foods, such as fresh-cut produce, soft cheeses, RTE refrigerated foods, or finfish.
- Training and Rollout: Train QA, production, and warehouse staff on mobile workflows and scanning, focusing on quick wins like faster record entry and deviation alerts.
- Scaling: Expand traceability across all products and sites, incorporating lessons learned and integrating supply chain partners.
- Continuous Improvement: Use trend data to identify recurring issues, refine CCPs, and improve operations. Regular mock recalls test system effectiveness and reveal gaps.
Effective change management includes defined roles, simple workflows, and visible benefits. When staff see mock recall times drop from days to minutes and audit prep shrink from 40 hours to 2, adoption follows naturally.
FoodReady AI supports data migration, user training, and alignment with certifications such as SQF Edition 9 or BRCGS Issue 9, and can be complemented by specialized HACCP consulting services for compliance to address whether having a HACCP plan is mandatory or voluntary in your specific segment.
Conclusion: Making HACCP Proactive with Digital Traceability
HACCP’s strength lies in the data supporting it. A well-written plan that cannot demonstrate effective implementation during audits or recalls offers false assurance. Food traceability software transforms HACCP from static documentation into a dynamic, continuously verified system where every lot, CCP check, and corrective action connects to consumer-bound products.
Aligning HACCP, FSMA 204 traceability, and GFSI expectations within a single platform reduces recall risks, accelerates investigations, and builds confidence with regulators, auditors, and key customers. Companies achieving this integration protect public health more effectively while reducing QA operational burdens.
With the January 2026 FSMA 204 compliance date approaching, implementing traceability solutions now, before crises or audits expose gaps, positions your operation to ensure food safety and maintain long-term compliance. Schedule a FoodReady AI demo or strategy session to assess your current HACCP and traceability setup and discover how an integrated platform can close gaps before deadlines.
FAQs
HACCP standards do not mandate software but require effective hazard control and documented records. However, regulators increasingly prefer digital systems for their reliability, legibility, and rapid retrieval. Paper records with high error rates and lengthy audit prep times no longer meet modern compliance expectations.
Complex operations, multiple sites, or FSMA 204-covered products often cannot meet 24-hour traceability and recall demands with paper alone. FoodReady facilitates smooth transitions from paper without disrupting HACCP certifications.
FoodReady exchanges data, item masters, purchase orders, production orders, inventory movements, shipping data, via APIs, file imports, or scheduled syncs with common ERP and WMS platforms. This prevents duplicate entry, maintains consistent lot codes and quantities, and reflects real-time production and inventory movements in traceability records.
Early integration workshops align data fields and workflows to minimize disruption.
Yes. The platform supports classical HACCP, FSMA Preventive Controls for Human Food, and blended programs used in SQF, BRCGS, and FSSC 22000. It integrates prerequisite programs such as GMPs, SSOPs, and allergen controls alongside quality control systems.
Users can define CCPs and preventive controls differently per product or facility while using a unified traceability and record-keeping backbone. FoodReady consultants include PCQI-qualified experts to align digital workflows with HACCP and FSMA PC requirements.
Mid-sized to enterprise food manufacturers, co-packers, and distributors with multiple lines, SKUs, or sites gain the most. Manual traceability becomes error-prone in these settings, and audits or recalls pose significant risks. The ROI from reduced audit prep, avoided recalls, and consumer confidence is substantial.
Smaller plants preparing for SQF/BRCGS, supplying national retailers, or handling high-risk or FSMA 204-listed foods also see strong returns. Modern traceability often justifies the investment. Understanding whether HACCP is mandatory or voluntary in your segment clarifies the business case. FoodReady scales from single-facility startups to multi-plant networks, with pricing based on complexity.
Timelines vary, but many companies have core HACCP forms, lot tracking, and traceability dashboards operational within 4-8 weeks, depending on data complexity and scope. For plants already under HACCP or SQF/BRCGS, digitalization mainly involves mapping existing processes to digital forms and training staff, aligning with audit cycles.
FoodReady’s recall simulation and audit-report tools help teams test readiness before auditors arrive. Mock recalls and report generation identify documentation gaps early, preventing surprises during inspections. This proactive approach builds consumer trust and protects against unsafe food reaching the market.








