Good Manufacturing Practices (GMP) lay the groundwork for safe and reliable production across the food, beverage, dietary supplements and nutraceutical industries. These regulations outline the bare minimum standards manufacturers must adhere to prevent contamination, fensure quality, and keep accurate records straight. Good Manufacturing Practice (GMP) is essentially a set of guidelines that ensure companies keep churning out products according to their quality standards – a crucial element in their regulatory compliance and quality control.
GMP guidelines include stuff like facility cleaning and equipment upkeep to employee hygiene, production controls and record keeping – the works. As these rules are universally recognised and enforced across global markets GMP compliance is now something every business expects, not just a competitive edge.
GMP software gives you a digital platform that helps manage these expectations. Rather than relying on pen and paper, spreadsheets, or a load of disconnected tools GMP software centralises all your documentation, standard operating procedures (SOPs), checklists, production records and compliance tasks in one place. Its easy to document and keep a record of every step of production – from receiving raw materials to checking batches and handling any problems that arise.
As a solid management tool that wont break the bank, GMP software helps businesses stay on top of quality standards, their quality and safety, and makes compliance processes smoother. By moving these processes into a tightly controlled digital environment, manufacturers reduce the risk of mistakes, improve their ability to track production and improve their day to day oversight of their operations.
Businesses need digital compliance now because regulatory bodies are demanding greater transparency, speed and accuracy than old manual systems can provide. FDA rules under 21 CFR Part 117, FSMA regulations like the Preventative controls framework and traceable requirements, USDA standards for meat and poultry facilities and EU rules on food safety and hygiene all mean companies are being pushed towards more reliable, technology driven processes. Digital solutions make it easier for a company to manage production processes on the fly, keep quality control on track and stay on top of compliance throughout production.
As regulations continue to evolve and the pressure on auditing compliance increases, digital GMP solutions give companies a sensible and scalable way to stick to their compliance obligations while keeping operations running smoothly.
Who Needs GMP Software?
A GMP solution is essential for any organization that produces, handles, or stores consumable goods.
While operational needs differ across categories, the common thread is the growing pressure to maintain accurate records, reduce compliance risks, and meet increasingly detailed regulatory expectations.

Below is a closer look at the sectors that benefit most from digital GMP systems and the specific challenges they face. With the right GMP tools, businesses can scale with confidence. This also helps ensure the business remains compliant with evolving regulatory requirements.
Food and Beverage Manufacturers
Food and beverage facilities manage complex production environments where sanitation, process control, and documentation must be consistently maintained. Manual logs make it difficult to track deviations, verify employee tasks, and prepare for audits. As 70% of manufacturers still enter data manually, digital GMP tools offer a clear path to stronger accuracy and compliance.
Digital GMP tools help standardize procedures, centralize logs, and ensure real-time visibility across production lines. This is imporant for maintaining product safety and passing regulatory inspections.
GMP software can also help monitor critical control points, providing real-time oversight to ensure food safety and compliance throughout the manufacturing process. Additionally, automated workflows streamline compliance tasks and reduce manual effort for food and beverage manufacturers.
Dietary Supplements & Nutraceuticals
Nutraceuticals and Supplement manufacturers operate under strict GMP requirements because their products are often concentrated and regulated closely for quality and labeling accuracy. Paper-based systems can’t reliably track batch formulas, weigh-up processes, or ingredient specifications. GMP software provides structured electronic batch records and traceability features that support both compliance and operational consistency. Additionally, GMP compliant software integrates design control, ensuring that product quality and regulatory compliance are maintained throughout the development and production process.
Co-Packers and Co-Manufacturers
These facilities must meet the expectations of multiple clients while adhering to their own regulatory obligations. Managing different formulations, packaging variations, and production runs often leads to paperwork overload. Digital systems streamline documentation, reduce miscommunication, and provide audit-ready records that clients and regulators expect.
Specialty Food Producers
Producers in categories such as ready-to-eat meals, frozen foods, bakery products, and shelf-stable packaged goods face continual pressure to document sanitation, allergen controls, and ingredient traceability. As over 40% of food recalls three years ago were due to undisclosed allergens. Tracking raw materials is essential to ensure compliance with GMP standards and to prevent contamination or other hazards in manufacturing. A single missed record can jeopardize certification or delay distribution. GMP software ensures consistent completion of checklists and automates reminders for critical tasks.
Warehouses, Distribution Centers, and 3PLs
While not always involved in production, storage and logistics operations must maintain GMP-level controls for temperature, cleanliness, pest management, and lot integrity. Tracking these activities on paper increases the risk of undocumented nonconformances. Digital compliance enables warehouses and 3PLs to maintain verifiable traceability and prove proper handling throughout the supply chain.
Pet Food & Animal Feed Manufacturers
Pet food and feed producers operate under stringent safety expectations that mirror those in human food manufacturing. Cross-contamination, ingredient integrity, and precise formulation control are critical. Without digital systems, documenting batch records, supplier verification, and sanitation activities becomes time-consuming and error-prone. GMP software ensures that every production run meets regulatory standards, supports rapid traceability during investigations, and reinforces consistent quality across product lines.
Cannabis & Hemp Processing Facilities
Cannabis and hemp manufacturers face a unique combination of GMP expectations, state-level regulations, potency testing requirements, and strict batch traceability demands. Paper systems struggle to manage extraction processes, formulation updates, packaging rules, and chain-of-custody tracking. GMP software centralizes documentation, improves traceability from seed to sale, and maintains the audit readiness required for licensing, inspections, and certification.
Medical Device Manufacturers
Medical device production requires rigorous documentation, process validation, and adherence to design controls to comply with standards such as FDA QSR and ISO 13485. Manual documentation increases the risk of gaps or inconsistencies that can delay approvals or trigger compliance findings. GMP systems ensure controlled processes, unified documentation, and verifiable quality management throughout the device lifecycle, from design and development to production and distribution.
Pharmaceuticals & Biotech
Pharmaceutical and biotech facilities must follow some of the most demanding GMP regulations in the world. Batch integrity, equipment validation, deviation management, and environmental monitoring all require precise documentation. Paper-based systems introduce unnecessary risk in such highly regulated environments. GMP software provides electronic batch records, automated audit trails, and robust document control to ensure compliance with FDA, EMA, and global regulatory expectations. This level of digital oversight strengthens quality systems and enables facilities to maintain consistent, repeatable production processes.
Digitize Your GMP Compliance Today
Replace manual logs and spreadsheets with one unified platform.
Rising Demand for Digital Compliance
The industry-wide shift toward digital GMP systems is driven by several converging pressures, including:
- More rigorous regulatory inspections and the need for regular inspections requiring fast, complete documentation.
- Customer and retailer expectations for full traceability and audit transparency.
- Growth in multi-product and multi-site operations that cannot rely on manual workflows.
- Higher risk profiles associated with allergen cross-contact, supplier variability, and global sourcing.
Together, these factors make GMP software a practical and increasingly necessary tool for modern food and supplement companies looking to maintain compliance, reduce operational friction, and scale with confidence.
What Are the Must-Have Features in Today’s GMP Compliance Software?
GMP compliance software brings structure, control, and traceability to compliance workflows. When evaluating solutions, it is essential to understand key GMP software features to ensure the platform meets your operational and regulatory needs.
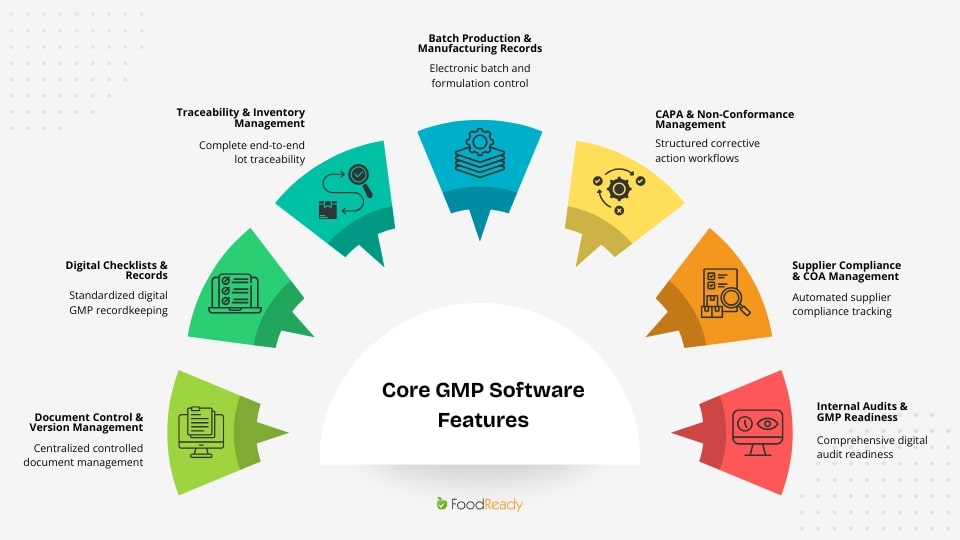
The following core features support the operational and regulatory requirements of food, beverage, supplement, and nutraceutical manufacturers.
Keeping Documents in Order – Document Control & Version Management
Effective compliance starts with accurate and accessible documentation. GMP platform centralizes SOPs, SSOPs, GMP policies, and related programs while enforcing strict version control. Users can manage controlled revisions, track approval workflows, and maintain training acknowledgments to ensure employees always work from the latest procedures.
Making Sure You Have the Right Records – Digital Checklists & Records
Daily compliance tasks rely on timely and complete documentation. Digital checklists replace paper logs for GMP daily activities, sanitation records, and pre-op and post-op inspections. These records are timestamped, standardized, and easy to retrieve during audits, reducing the risk of missing or inconsistent data.
Keeping a Handle on Your Inventory – Traceability & Inventory Management
Traceability is a core requirement for GMP and FSMA compliance. GMP software supports lot tracking and full lot genealogy from receiving through production and shipping. Real-time visibility into materials and finished goods strengthens recall readiness and helps companies meet industry and regulatory traceability expectations.
Streamlining Batch Production – Electronic Batch Records
GMP software provides a structured environment for managing batch operations. Electronic Batch Records (EBR), Bill of Materials (BOM) management, and formulation controls ensure consistency across production runs. Waste tracking and yield management features help facilities monitor efficiency and identify deviations in real time.
Catching Issues Before They Become Problems – CAPA & Non-Conformance Management
When deviations occur, including non conformance issues that impact quality management, corrective and preventive action (CAPA) tools streamline documentation and resolution. The system guides users through standardized corrective actions and root cause analysis workflows, ensuring issues are investigated, addressed, and verified according to GMP expectations.
Managing Suppliers with Ease – Supplier Compliance & COA Management
Managing supplier performance and documentation is essential for compliant operations. GMP software maintains approved supplier lists, tracks specifications, and stores Certificates of Analysis (COAs). Automated alerts notify users of missing or expired documents, helping prevent unapproved materials from entering production.
Staying Audit-Ready, All Year Round – Internal Audits & GMP Readiness
To support ongoing compliance, GMP software includes tools for internal audit management. Preloaded GMP checklists, audit scheduling capabilities, and digital evidence collection help facilities stay audit-ready throughout the year and maintain consistent oversight of their programs and procedures.
What Are the Benefits of Using GMP Software?
Digital workflows make a huge difference in terms of consistency and efficiency across the facility. By getting rid of paper-based bottlenecks, teams reduce manual errors, eliminate duplicate records, and streamline documentation.
Standardized GMP processes make it easier for employees to follow procedures, complete required logs, and maintain production continuity. And with a user-friendly interface, adoption and operational efficiency are no problem at all – ensuring that staff can easily navigate the system and comply with GMP requirements. Audit preparation is also much faster, because records are automatically organized, timestamped, and accessible in one system.
1. Operational Benefits
Digital workflows make a huge difference in terms of consistency and efficiency across the facility. By getting rid of paper-based bottlenecks, teams reduce manual errors and eliminate duplicate records with a document manag, and streamline documentation.
Standardized GMP processes make it easier for employees to follow procedures, complete required logs, and maintain production continuity. And with a user-friendly interface, adoption and operational efficiency are no problem at all – ensuring that staff can easily navigate the system and comply with GMP requirements.
Audit preparation is also much faster, because records are automatically organized, timestamped, and accessible in one system.
2. GMP Compliance Benefits
GMP software improves regulatory alignment by providing real-time visibility into deviations and non-conformances. Supervisors can review issues immediately, verify corrective actions, and maintain a complete audit trail.
A GMP document management system simplifies the inspection process by presenting auditors with organized, validated records. This structure supports GMP certification and regulatory efforts, including cGMP, SQF, BRCGS, and HACCP, by ensuring each requirement is consistently documented and traceable.
3. Financial / ROI Benefits
Adopting GMP software has a real impact on your bottom line. Reduced waste and rework occur when batch records, ingredient usage, and process controls are monitored accurately. Companies also lower recall risk through stronger traceability and faster lot tracking.
And, let’s not forget about the administrative costs – which decline because teams spend less time searching for paperwork or preparing audit binders.

Over time, these combined efficiencies reinforce the return on investment by improving reliability, productivity, and overall compliance performance.
Using GMP Software to Prepare for Audits
Instead of relying on paper binders or manually assembled spreadsheets, the sa digital sytem automatically stores logs, corrective actions, complaints handling records, production records, and supplier documentation in categorized folders.
This structure allows auditors to locate required information quickly and reduces the risk of missing or incomplete records.
How the Software Organizes Records for Auditors?
The platform connects daily activities, such as sanitation logs, batch production steps, equipment checks, and material receiving, to corresponding GMP categories.
Each entry is timestamped, assigned to a user, and linked to supporting evidence when needed.
During an audit, facilities can provide read-only access or generate comprehensive reports that reflect real-time compliance status. This transparency helps inspectors verify processes without digging through manual paperwork.
Typical Audit-Ready Document Structure
Most GMP software solutions use a standardized hierarchy to align with regulatory and certification requirements. A typical audit-ready structure includes:
- Programs and Policies: SOPs, SSOPs, GMP policies, and prerequisite programs
- Daily Records: sanitation logs, inspections, production entries, and deviation reports
- Corrective Actions: CAPA documentation with root cause analysis
- Traceability Files: lot tracking, COAs, supplier approvals, and recall readiness documents
- Training Records: employee certifications, acknowledgments, and retraining logs
This system ensures the entire documentation package is complete and easy for auditors to follow.
A Few Audit Preparation Use Cases
GMP software supports a wide range of regulatory and certification audits. Companies preparing for cGMP certification can quickly produce batch records, sanitation logs, and training files that demonstrate consistent compliance.
During FDA inspections, digital records show adherence to 21 CFR Part 117 through clear documentation of preventive controls, monitoring activities, and corrective actions.
For GFSI benchmarked schemes such as SQF or BRCGS, the software helps facilities maintain evidence of implementation for each code element, making certification audits more predictable and less disruptive.
By centralizing records and enforcing structured documentation, GMP software shortens audit timelines and strengthens the reliability of every compliance-related activity.
In the pharmaceutical industry, GMP software is essential for audit preparation, as it ensures compliance with strict regulatory standards and supports quality and safety requirements.
See How Digital GMP Systems Work in Real Operations
Customize the digital solution to
your exact business operations
How to Choose the Right GMP Software?
Selecting the right solution requires a structured evaluation process that considers both operational needs and long-term compliance goals. The ideal platform should improve daily workflows, support regulatory requirements, and scale as the business grows.
It is also crucial to choose a solution that integrates seamlessly with your existing systems, ensuring flexibility and compatibility for your operations.
Key Evaluation Criteria
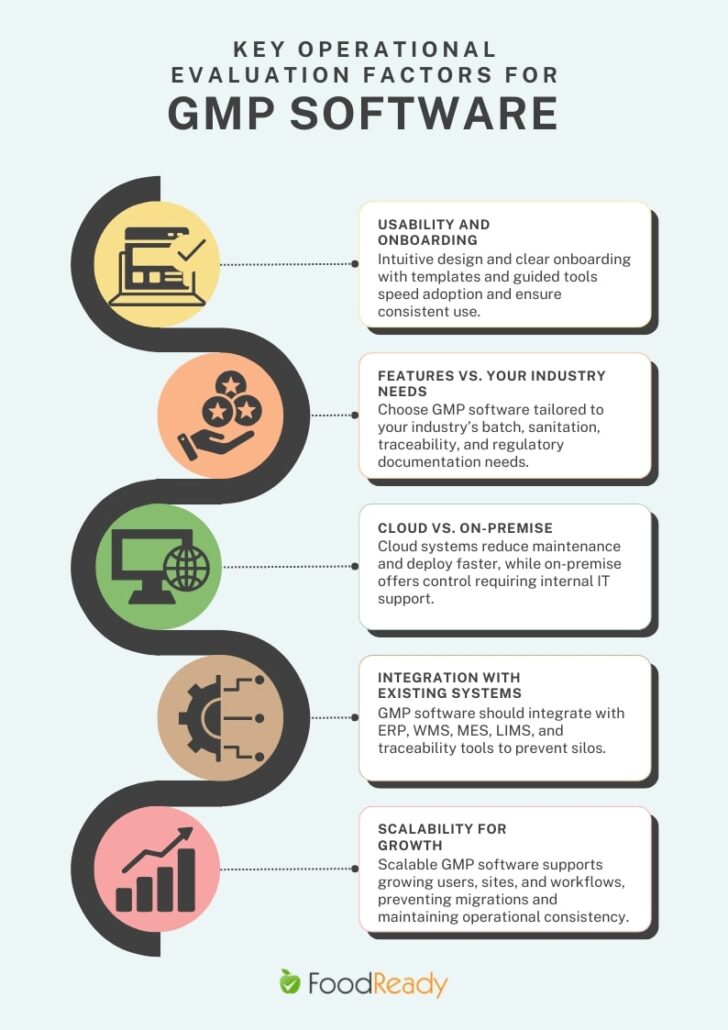
1. Usability and Onboarding
Teams adopt software more effectively when the interface is intuitive and the onboarding plan is clear. Strong training resources, preloaded templates, and guided setup tools reduce implementation time and support consistent use across departments.
2. Features vs. Your Industry Needs
Not all GMP systems serve every segment equally. Food, beverage, supplement, and nutraceutical operations require features such as batch records, sanitation logs, traceability tools, and supplier compliance modules. The software should align directly with your regulatory, production, and documentation requirements.
3. Cloud vs. On-Premise
Cloud platforms offer easier updates, faster deployment, and lower maintenance costs, while on-premise solutions provide more control over infrastructure but require internal IT resources. The right choice depends on your operational structure and security expectations.
4. Integration with Existing Systems
GMP software should integrate with ERP, WMS, MES, laboratory systems, or traceability tools to avoid data silos. Seamless integration ensures consistent lot tracking, synchronized production records, and accurate inventory data.
5. Scalability for Growth
As production lines expand or new facilities come online, the system should support additional users, sites, and workflows. Scalable GMP software prevents costly migrations and maintains consistency across operations.
What Questions to Ask The Vendors?
When evaluating vendors, targeted questions help determine whether the platform meets compliance and operational expectations:
- “How does your platform support FDA 21 CFR Part 117?”
- “Does the software support lot tracing and EBRs?”
- “Are templates included for GMP programs and checklists?”
- “How does role-based access control work?”
Clear, detailed answers to these questions indicate how well the software will support regulatory compliance, production visibility, and long-term operational stability.
Top GMP Software for Food, Beverage & Supplement Manufacturers
1. FoodReady
Platforms: Web, Android, iOS
Free version: By Invite
Pricing: Contact FoodReady
Why use FoodReady?
FoodReady is an end-to-end food safety and GMP compliance platform built specifically for food and beverage, supplement, and co-manufacturing operations. It combines digital GMP workflows, full traceability, batch production tools, and expert consulting support in one system, making it easier for companies to manage compliance from the production floor to audit preparation. The software includes prebuilt templates for GMP, HACCP, SQF, BRCGS, and FSMA programs, accelerating onboarding and reducing manual documentation. FoodReady stands out for its integrated recall module, robust inventory and traceability features, and the option to work directly with food safety consultants for certification readiness.
FoodReady is ideal for small to mid-sized manufacturers that need a practical, scalable compliance solution without the complexity of enterprise-level systems.
2. SafetyCulture
Platforms: Web, Android, iOS
Free version: Available
Pricing: Contact SafetyCulture
Why use SafetyCulture?
SafetyCulture offers robust inspection and auditing tools that streamline GMP documentation and internal verification tasks. It allows teams to build and use standardized checklists, capture photos as evidence, and generate audit reports instantly. The platform is highly flexible and works well for dispersed teams or multi-location operations. Its mobile-first design supports real-time data capture on the production floor.
Key Features:
- Checklists and Templates
- Reporting and Analytics
- Incident Reporting
3. Enterprise 21
Platforms: Web
Free version: Unavailable
Pricing: Contact TGI
Why use Enterprise 21?
Enterprise 21 by TGI includes full ERP capabilities with embedded GMP-related controls. The system supports audit trails, electronic signatures, and controlled documentation to maintain data integrity. Its forward and backward lot traceability helps facilities manage recalls and verify product genealogy at any point. Enterprise 21 is particularly suited for manufacturers that need GMP features within a broader ERP environment.
Key Features:
- Lot Traceability
- Shelf Life Management
- Inventory Management
4. ETQ Reliance
Platforms: Web, iOS, Android
Free version: Unavailable
Pricing: Contact ETQ Reliance
Why use ETQ Reliance?
ETQ Reliance is a comprehensive QMS platform with strong workflow automation, making it valuable for GMP compliance and audit management. It supports controlled documentation, CAPA, risk management, and supplier oversight in a unified system. Its configurability allows organizations to tailor workflows to match their GMP programs, reducing manual routing and approval steps. ETQ is widely adopted across regulated industries due to its validated, compliance-focused structure.
Key Features:
- Audit Management
- Food Safety Management
- Checklists
- Document Control
5. MasterControl
Why use MasterControl?
MasterControl is designed for life sciences and regulated manufacturing environments, offering deep functionality for document control, CAPA, training, audits, and change management. The platform is validated against FDA and ISO requirements, ensuring strong data integrity and compliance coverage. It supports large, multi-department organizations with complex regulatory oversight needs. While implementation is more intensive, MasterControl delivers extensive tools for maintaining traceability across every workflow.
Key Features:
- Implementation and Validation Software
- Process Management
- Centralized Information Management
6. Tulip
Why use Tulip?
Tulip is a no-code manufacturing execution and compliance platform that helps digitize batch records, work instructions, and production data. Teams can build custom workflows for training checks, sanitation tasks, and audit documentation without engineering support. Its ability to connect to equipment and capture real-time data strengthens process visibility and deviation detection. Tulip is ideal for operations seeking configurable tools that can adapt across diverse facilities.
Key Features:
- Streamlined Compliance
- Hygiene and Sanitation Workflows
- Automated Documentation
7. ABB
Why use ABB?
ABB provides automation and process control tools used widely in industrial food and beverage operations. Its compliance modules support training management, change control, and environmental data capture. ABB integrates directly with production equipment, enabling real-time monitoring of GMP-critical parameters. This makes it suitable for large facilities that require both automation and structured compliance oversight.
Key Features:
- Document and Training Management
- Quality Control and Quality Assurance
- Compliance Monitoring and Change Control
What Are the Common Mistakes Manufacturers Make When Implementing GMP Software?
Successful GMP software adoption requires careful planning and coordination across departments. Many implementation challenges occur not because of the software itself, but because of gaps in preparation and execution. The following mistakes are among the most common and can delay full adoption or reduce the system’s effectiveness.
Underestimating Training Needs
Teams often assume that users will naturally adapt to new digital workflows, but GMP software introduces new steps, approvals, and documentation rules. Insufficient training leads to inconsistent recordkeeping, incomplete logs, and reliance on old habits. Structured onboarding, clear user instructions, and ongoing reinforcement are essential for long-term success.
Not Customizing Templates to Real Operations
Most platforms include prebuilt templates for GMP logs, sanitation forms, and audit checklists. When facilities don’t customize these templates to match their actual processes, employees either skip required fields or add information elsewhere. Tailoring templates ensures the system reflects real workflows and supports accurate, compliant documentation.
Poor Data Migration Planning
Historical records, supplier documentation, and specifications must be brought into the system correctly for it to be fully functional. Unorganized data migration creates gaps in traceability and confusion during audits.
A clear plan, which includes defining what to import, who owns each dataset, and how it should be structured, prevents long-term issues.
Not Assigning Internal Ownership
GMP software needs internal champions who oversee configuration, user access, and ongoing improvements. Without defined ownership, tasks such as updating SOPs, revising forms, or reviewing deviations can become inconsistent. Assigning a dedicated compliance or quality lead ensures accountability and maintains system reliability over time.
Best Practices for Successful GMP Software Adoption
Effective implementation of GMP software requires structured planning and consistent execution. The following practices help organizations achieve reliable adoption, maintain compliance, and integrate digital workflows into daily operations.
Establish a Dedicated Implementation Team
Assign clear roles for configuration, data migration, user onboarding, and ongoing maintenance. A defined team ensures accountability and prevents gaps in system ownership. In most facilities, this includes quality, operations, and IT stakeholders working together.
Customize Workflows and Templates Early
Adapting prebuilt templates to match your actual production and compliance processes is essential. Early customization reduces user confusion, minimizes workarounds, and ensures the system reflects real operational requirements. This step also strengthens documentation consistency across shifts.
Integrate GMP Software With Existing Systems
Connecting the platform to ERP, WMS, traceability, or laboratory systems prevents data silos and ensures accurate lot tracking. Integration also reduces manual entry and improves production visibility, which supports faster decision-making during audits or investigations.
Conduct Phased Training and Verification
Training should occur in structured phases rather than one-time sessions. Begin with core users, verify proper system use, and expand training to additional roles once workflows are stable. Periodic retraining reinforces compliance expectations and improves record accuracy.
Here Are the Key Steps for Long-Term Success
- Align software configuration with actual processes
- Validate workflows before going live
- Review documentation completeness regularly
- Adjust forms and tasks as operations evolve
Implementing these practices helps manufacturers achieve reliable digital compliance and maintain audit-ready records throughout the year.
Future-Proof Your GMP Program
Adopt a scalable system built for food, beverage, and supplement manufacturers.
Emerging Trends in Good Manufacturing Practice Digital Area
GMP software is evolving quickly as regulatory expectations rise and manufacturers look for more efficient ways to manage compliance. Several key trends are shaping the next generation of digital GMP systems and influencing how food, beverage, supplement, and co-manufacturing operations approach compliance and production oversight.
AI-Assisted Compliance and Documentation
AI-driven tools are increasingly being embedded into GMP platforms to automate repetitive tasks such as filling out logs, identifying missing data, suggesting corrective actions, and analyzing patterns in deviations. These capabilities help reduce human error, support faster verification, and give quality teams real-time insights into potential compliance risks.
Integrations and Internet of Things
Manufacturers are adopting connected sensors and automated data capture to reduce manual entries. Integration with scales, thermometers, environmental monitors, and production equipment ensures data flows directly into GMP records. This enhances accuracy and strengthens audit defensibility by providing timestamped, equipment-generated data.
Real-Time Traceability and FSMA 204 Alignment
Traceability systems are moving toward instant lot visibility across suppliers, production, warehousing, and distribution. Platforms are integrating advanced lot genealogy, automated event logging, and faster recall simulation capabilities. These features support compliance with FSMA 204 and other global traceability requirements.
Configurable, No-Code Workflows
More GMP systems now allow quality and operations teams to build or modify workflows without IT involvement. No-code tools make it easier to adapt forms, checklists, routing steps, and approval processes as operations evolve. This flexibility reduces reliance on costly custom development.
Mobile-First GMP Execution
As production-floor teams move away from clipboards, mobile devices have become the primary interface for completing GMP tasks. Modern systems prioritize offline functionality, photo-based evidence capture, and simplified mobile checklists to ensure consistent recordkeeping across all shifts and environments.
Integrated Quality + Production Platforms
Manufacturers increasingly prefer systems that combine good manufacturing practice, production, inventory, and traceability into a unified platform. Integrated environments prevent data silos, improve decision-making, and support continuous improvement by linking quality events with operational outcomes.
Cloud Adoption and Remote Audit Readiness
Cloud-based GMP systems are now standard, offering secure access from any location and simplifying multi-site management. Remote audit tools, such as document portals, screen-sharing capabilities, and automated compliance reports, allow facilities to complete portions of external audits without onsite inspectors.
Conclusion
GMP software has become an essential component of modern food, beverage, supplement, and co-manufacturing operations. As regulatory expectations increase and supply chains grow more complex, manufacturers can no longer rely on paper-based systems or disconnected tools to manage compliance. Digital platforms offer the structure, traceability, and real-time visibility needed to maintain consistent GMP controls, reduce deviation risks, and stay audit-ready throughout the year.
By selecting a system that aligns with operational requirements, integrating it into existing workflows, and committing to proper onboarding, companies can significantly improve documentation accuracy and operational efficiency.
AI-driven insights, IoT connectivity, and advanced traceability are enhancing the role of GMP software and shaping the future of compliant production.
For organizations aiming to strengthen food safety, streamline processes, and support continuous improvement, a well-implemented GMP software platform is now one of the most strategic investments they can make.
FAQs
GMP software is a digital system that manages Good Manufacturing Practices by organizing documentation, standardizing workflows, and ensuring consistent compliance across production, sanitation, training, and quality processes.
Any food, beverage, supplement, or nutraceutical manufacturer that must follow GMP, cGMP, or GFSI standards benefits from GMP software. Co-packers, warehouses, and distributors also use it to maintain traceability and proper handling practices.
GMP software centralizes all records into a structured, searchable system. This include SOPs, sanitation logs, batch records, CAPA files, and training documentation. This reduces audit prep time and ensures auditors can access complete, validated records.
While not explicitly required, digital systems help meet FDA expectations under 21 CFR Part 117 by improving documentation accuracy, traceability, and preventive controls management.
ERP focus on operational planning, inventory, and financials, while GMP focuses on food safety, compliance, documentation, and quality control. Many businesses use both, integrating GMP software with their ERP.
Yes. Most GMP platforms provide electronic batch records that capture ingredient usage, process steps, operator signatures, and deviations, offering stronger traceability than paper systems.
Modern GMP platforms include lot tracking, lot genealogy, receiving logs, and recall readiness tools that support FSMA 204 requirements for high-risk foods and traceability events.
Implementation time varies by facility size and complexity but typically ranges from a few weeks to several months. Early planning, data preparation, and proper training shorten the timeline.
Many systems support integration with IoT sensors, scales, thermometers, lab instruments, or MES systems to automate data capture and reduce manual entries.
By enforcing required fields, prompting timely checks, and providing alerts for out-of-spec results, GMP software reduces the chance of incomplete or missed documentation and enables earlier detection of issues.
Yes. Small operations often see significant time savings and improved audit readiness by digitizing logs, SOPs, and checklists. Many platforms offer scalable pricing suitable for smaller facilities.
Key features include document control, digital checklists, traceability, supplier management, batch records, CAPA workflows, and audit tools. Ideally, the system should also support mobile use and integration with existing software.








