cGMP Consultant
Rapidly obtain cGMP certification with FoodReady.
- We help you meet all cGMP requirements & we prepare you & schedule a successful cGMP audit to obtain cGMP Certification.
- Expert cGMP Consulting Services for Nutraceutical, Food, Cannabis, OTC, Supplement, Packaging and Cosmetic Manufacturers.
- Customized development and implementation of cGMP Compliant Quality Systems.
- Full support with cGMP Documentation Control and Audit Readiness.
- Ensure product quality standards meet FDA and other regulatory requirements of governments and industry.

Leading Companies Trust FoodReady






Meet the Lead of Our Expert cGMP Consultant Team
Our Chief Food Safety Officer, Dave Seddon, is a longtime food safety auditor who has audited over 500 manufacturers for SQF certification, as well as GMP certification. Dave was named a finalist for the SQF auditor of the year by the SQF Institute.

Engage Our cGMP Consultant to Guarantee cGMP Compliance
We will deliver the best job with the best team
Accelerated Certification:
Our cGMP consultant will streamline compliance by identifying and addressing gaps in your current system, making certification achievable.
Customized Compliance:
Our cGMP consultant will tailor-make your cGMP plan to specific company operations, ensuring sustainable systems aligned with regulatory expectations
Enhanced Documentation:
Our cGMP consultant will guide the creation and standardization of critical records for successful inspections and certification maintenance.
Employee Training:
Our cGMP consultant will ensure your team understands responsibilities and regulations through targeted training, improving inspection performance.
Reduced Risk & Market Access:
Our cGMP Consultant will teach you how to minimize violations and recalls, enhancing credibility for access to regulated markets and growth

Hear It From Real People
Grow Your Food Business With Our cGMP Program
Let our team help you ace your next cGMP certification audit! We’ll take care of scheduling, customizing your cGMP Program, reviewing documentation, and developing cGMP plans & checklists. We also perform a gap assessment to identify areas for improvement and create necessary documentation for a successful audit. Trust us to make your audit process a breeze.
Build a Compliant cGMP Program Fast with Help from Industry Experts
Our cGMP consultants specialize in getting your cGMP program up and running fast! Don’t waste time trying to write it yourself. Leave it to the experts. With our team of professionals, you’ll have a certified cGMP program tailored to meet your needs in no time.
- Improved food safety and product quality
- Compliance with cGMP regulations and standards
- Reduced risk of cGMP non-compliance, fines, and penalties
- Improved cGMP risk management and hazard control
- Customized cGMP program design to meet your specific needs and goals
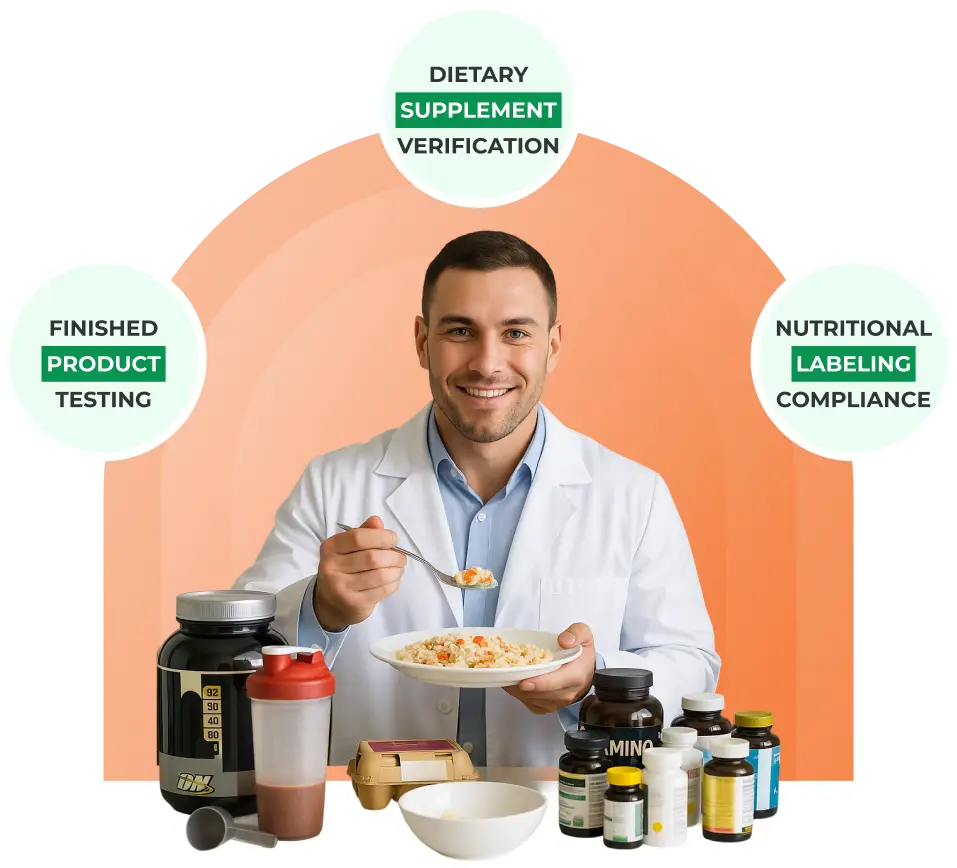
We Assist You With Implementing Your cGMP Program
We assist you in implementing your cGMP program. Our experts will team up with you to actualize your cGMP program, ensuring you ace your next cGMP certification audit. But wait, there’s more! Not only do we offer top-notch cGMP consultants to support you, but we’ve also got a user-friendly mobile app that makes managing all your cGMP programs and food safety records a breeze! Stay on top of everything from checklists to traceability and environmental monitoring — all at your fingertips!

Why Use FoodReady AI?
With searchable archives of traceability records, master sanitation logs & SOPs
Enhanced pharmaceutical and food reputation with properly maintained documentation
Reduced risk of recalls and foodborne illness with full compliance to cGMP rules and regulations
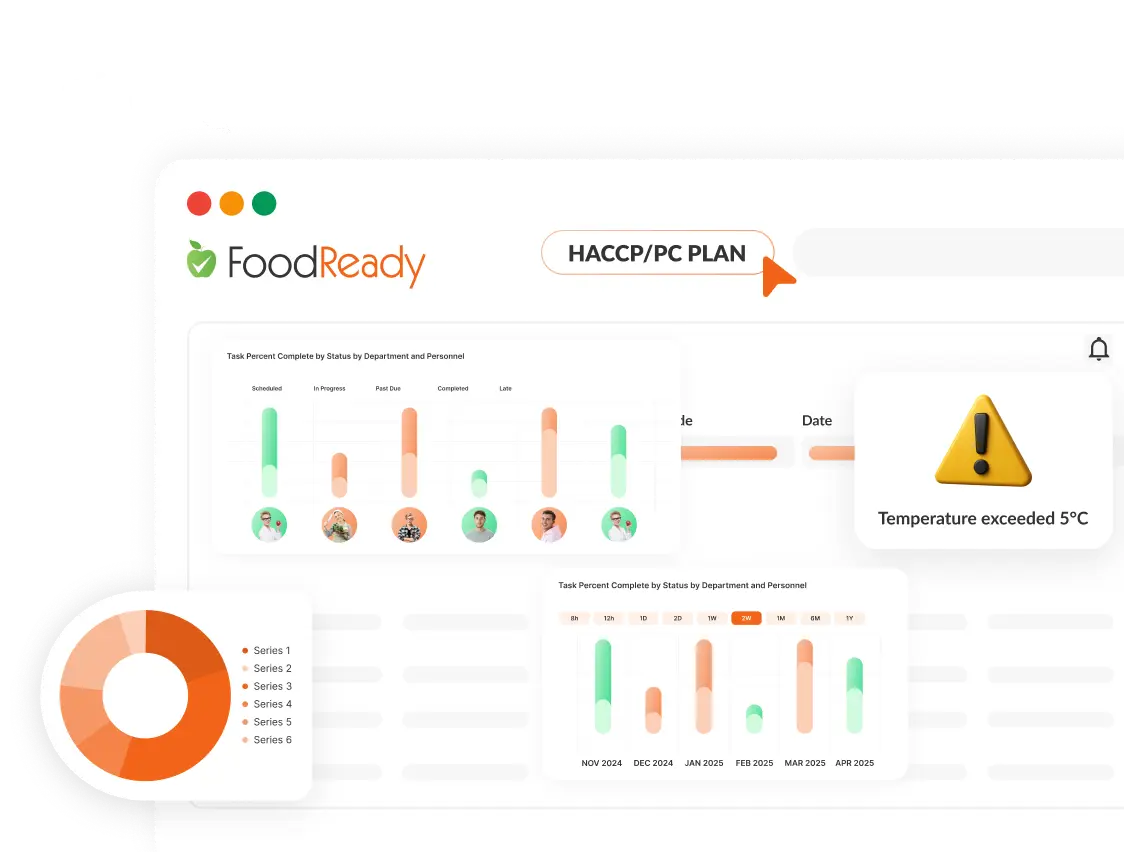
How Can Our AI Food Safety Management System Help You?
Our AI-Powered Food Safety Management System is more than just a compliance tool – it’s an intelligent system designed to streamline and strengthen your food safety and operational processes. The AI gives you great suggestions, and it’s all built-in with food safety smart tips from experts tailored for different kinds of food industries.
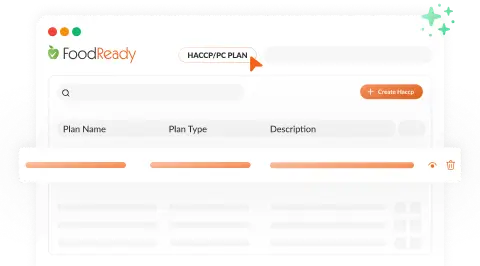
Rapid AI HACCP Plan Creation
FoodReady’s AI HACCP Builder offers pre-built templates, cutting HACCP plan development from hours to minutes.
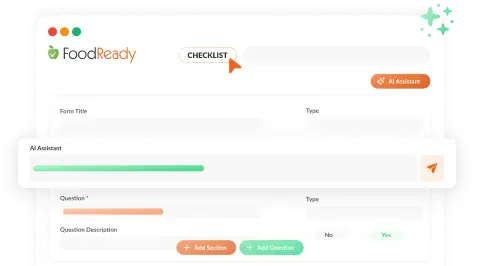
AI-Generated SOPs & Checklists
FoodReady’s AI Assistant creates and automates SOPs and checklists, detecting missed tasks and ensuring protocol compliance
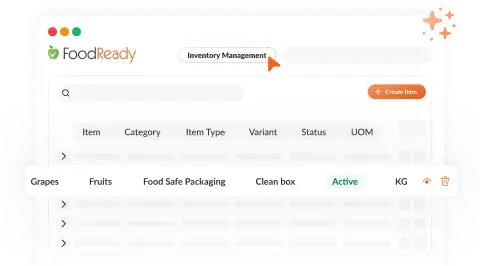
Unified Batch & Inventory Management
Our AI Assistant offers real-time tracking of production, inventory, and costs, enhancing efficiency and reducing compliance problems
Complete Traceability & Recall Readiness
FoodReady’s AI provides full traceability by linking ingredients to final products, facilitating rapid recalls and efficient audits
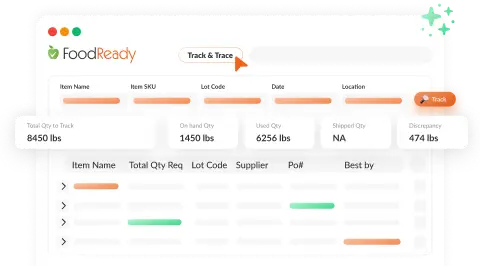
Automated Supplier Compliance
Reduce manual effort and increase transparency with AI-powered automatic management and updates of supplier documents
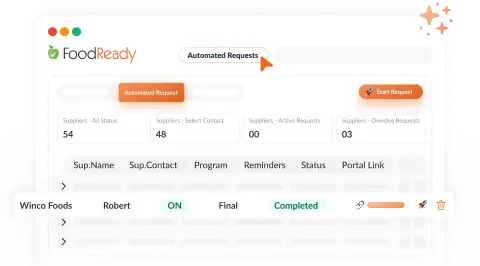
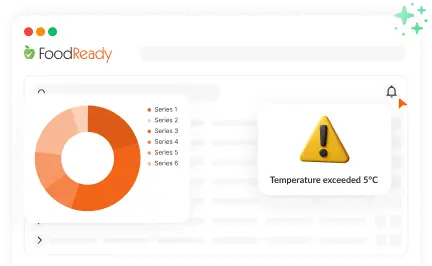
Live Monitoring & Instant Alerts
AI-powered dashboards offer real-time KPI monitoring and safety incident alerts for timely intervention.
Our Process for cGMP Compliance



Gap Assessment
We review the client’s existing documentation and facility to identify where the gaps exist and advise how to close the gaps to pass a successful cGMP inspection and comply with cGMP regulations.
HACCP Plan Document Development
This step is where we develop any new documentation, including new or updated HACCP Plans and new Sanitation Standard Operating Procedures (SSOPs), and create your sanitation management plan for food traceability according to cGMP regulations.
Environmental Monitoring Program
This step is where we develop your environmental monitoring program, including Listeria detection. FoodReady also has an easy-to-use mobile app that makes it easier to manage your environmental monitoring program.
We Can Help You Develop Your Food Safety Documentation
- Eliminate Paperwork.
- Increase Efficiency.
- Reduce Potential Hazards.
- Minimize Operational Risks.
- Train New Staff Faster.
Frequently Asked Questions
A cGMP consultant helps companies comply with FDA Good Manufacturing Practices by auditing facilities, developing SOPs, training staff, and ensuring regulatory readiness. Their focus is on product consistency, safety, and quality across manufacturing. They support validation, risk assessment, and CAPA implementation, primarily for food, pharma, and supplement industries seeking to meet legal and industry safety standards.
A cGMP consultant can conduct gap assessments, review documentation, train employees, and implement corrective actions to ensure compliance with GMP standards and a successful audit outcome.
Yes, cGMP consultants can help integrate cGMP standards into broader food safety certifications like SQF, BRC, or GFSI to streamline compliance efforts and enhance your overall food safety management system.
A cGMP consultant can assist in developing essential components such as sanitation practices, documentation control, training programs, production process monitoring, and corrective action procedures to ensure compliance with regulatory standards.
Yes, cGMP consultants provide ongoing support by conducting periodic reviews, updating programs to meet changing regulations, and ensuring that your practices remain compliant and audit-ready.

Speak with our
Expert Consultants
Get a Free Consultation With Our Food Safety
and Quality Experts Today!
Get in Touch with Us
We are always here to help

